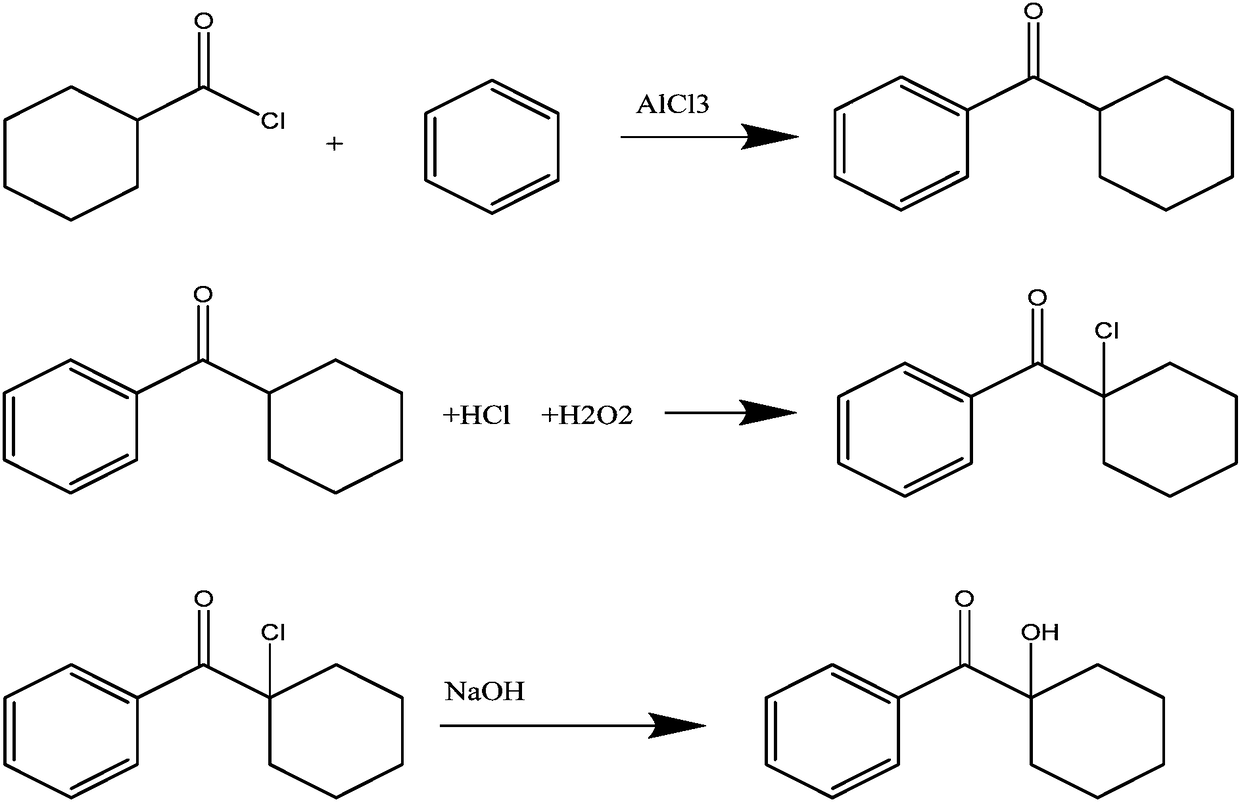
Synthetic method of photoinitiator 1-hydroxycyclohexyl phenyl ketone
A technology of hydroxycyclohexyl phenyl ketone and cyclohexyl phenyl ketone, which is applied in the field of preparation of chlorinated hydrolysis, can solve the problems of acid waste water and achieve the effects of reduced discharge, less loss and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Add anhydrous AlCl to a 500ml four-neck flask equipped with stirring 3 80.0g (0.6mol), add 235g (3mol) of benzene in an ice-water bath, drop it below 0°C, add 73.3g (0.5mol) of cyclohexanoyl chloride (constant pressure funnel) to the bottle dropwise, drop Keep the temperature at 0-5°C during the addition, and the dropwise addition time is about 30 minutes. After the dropwise addition, slowly raise the temperature to 78°C, and react under reflux for 6 hours. After the reaction, pour the reactant into 360g of 20% diluted In hydrochloric acid (1.97mol), stir well and then separate the phases, take the upper organic phase and carry out vacuum distillation to recover benzene, after benzene is distilled off, 85.8g (A) of light yellow solid (content 92%, 0.42mol) is obtained, and the molar yield is 84 %. The acid water in the lower layer was distilled under reduced pressure, and 300 g (content 23%) of hydrochloric acid aqueous solution (B) was distilled off.
[0017] Add (A...
Embodiment 2
[0019] Add anhydrous AlCl to a 500ml four-neck flask equipped with stirring 3 80.0g (0.6mol), add 235g (3mol) of benzene in an ice-water bath, drop it below 0°C, add 73.3g (0.5mol) of cyclohexanoyl chloride (constant pressure funnel) to the bottle dropwise, drop Keep at 0-5°C while adding, add about 30 minutes, slowly heat up to 78°C after dropwise addition, react under reflux for 6 hours, after the reaction, cool down to about 0°C, add 360g of 20% dilute hydrochloric acid (1.97mol) Drop into the reactant, stir well and then separate the phases. Take the upper organic phase and carry out vacuum distillation to recover benzene. After benzene is distilled off, 83.2 g of light yellow solid (A) (content 91.5%, 0.4 mol) is obtained, and the molar yield is 80%. . The acid water in the lower layer was distilled under reduced pressure, and 313 g (content 22%) of hydrochloric acid aqueous solution (B) was distilled off.
[0020] Add (A) 83.2g (content 91.5%, 0.4mol), 1,1-dichloroeth...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com