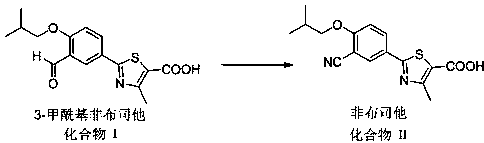
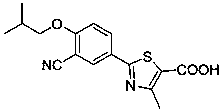
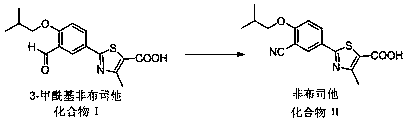Synthesis method for transforming impurity 3-formyl febuxostat into febuxostat
A formyl febux and synthesis method technology, applied in the field of pharmaceutical chemical synthesis, can solve problems such as unreasonable utilization, environmental pollution, difficult control of diazotization reaction, etc., and achieve convenient operation, high yield, suitable for large-scale The effect of large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add 3.19 g (0.01 mol) 3-formyl febuxostat, 2.3 g (0.02 mol) azidotrimethylsilane, and 50 ml ethyl acetate to a 100 ml three-necked flask equipped with a thermometer and mechanically stirred. , slowly added 6.88 g (0.04mol) of p-toluenesulfonic acid, heated in a water bath, raised the temperature to 40 °C, and kept the temperature for 12 hours. The reaction was monitored by TLC. 2.59 g of febuxostat was obtained by crystallization, with a yield of 82% and a content of 97.9%.
Embodiment 2
[0028] Add 3.19 g (0.01 mol) 3-formyl febuxostat, 3.94 g (0.02 mol) p-toluenesulfonyl azide, 50 ml ethyl acetate, respectively, to a 100 ml three-neck flask equipped with a thermometer and mechanical stirring. Slowly add 6.88 g (0.04mol) of p-toluenesulfonic acid, heat in a water bath, raise the temperature to 40 °C, keep the temperature for 12 hours, and monitor the reaction by TLC. After the reaction, distill off the ethyl acetate in the reaction solution and recrystallize with ethanol 2.56 g of febuxostat was obtained with a yield of 81% and a content of 98.0%.
Embodiment 3
[0030] Add 3.19 g (0.01 mol) 3-formyl febuxostat, 1.3 g (0.02 mol) sodium azide, and 50 ml ethyl acetate to a 100 ml three-necked flask equipped with a thermometer and mechanical stirring, and slowly add 6.88 g (0.04 mol) of p-toluenesulfonic acid, heated in a water bath, raised the temperature to 40 ° C, kept the temperature for 12 hours, and monitored the reaction by TLC. After the reaction, distilled off the ethyl acetate in the reaction solution, and recrystallized with ethanol to obtain non- Buxostat 2.81 g, yield 89%, content 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com