Medicine for treating pancreatitis, and preparation method thereof
A pharmacy and compound technology, applied in the field of medicines for the treatment of pancreatitis and their preparation, can solve problems such as being unable to meet actual needs and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
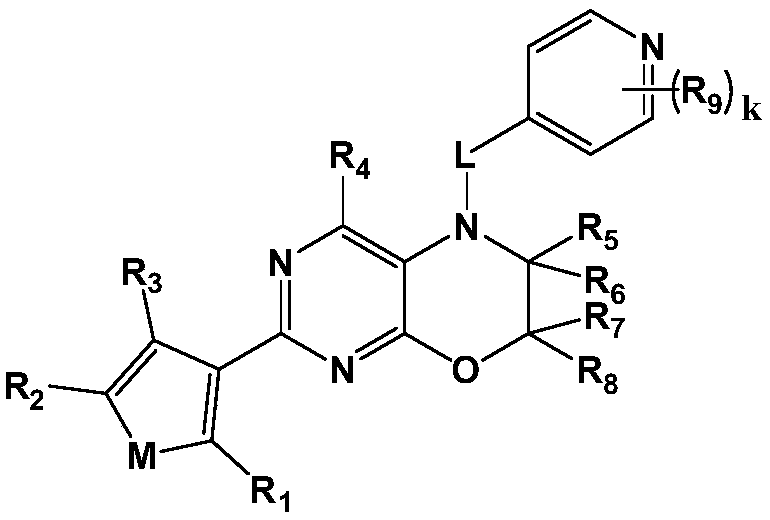
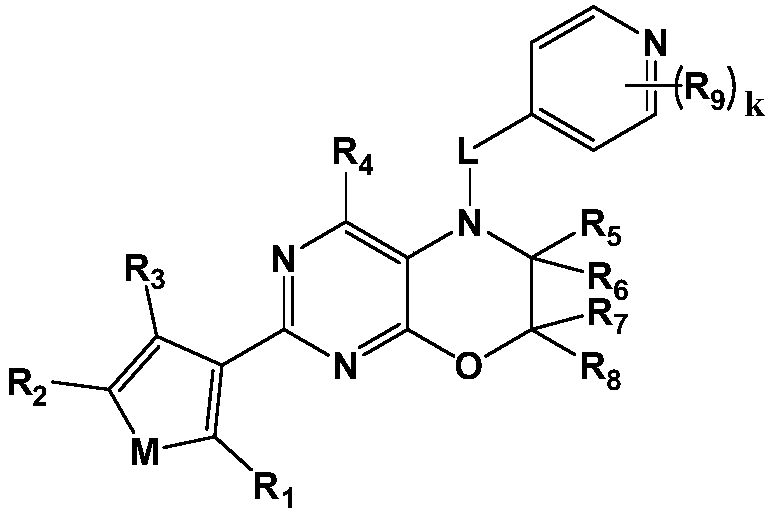
[0085] Embodiment 1: synthetic compound I-1
[0086] 5-((2-methoxypyridin-4-yl)methyl)-2-(thien-3-yl)-6,7-dihydro-5H-pyrimidine[4,5-b][1,4 ] Zinc
[0087]
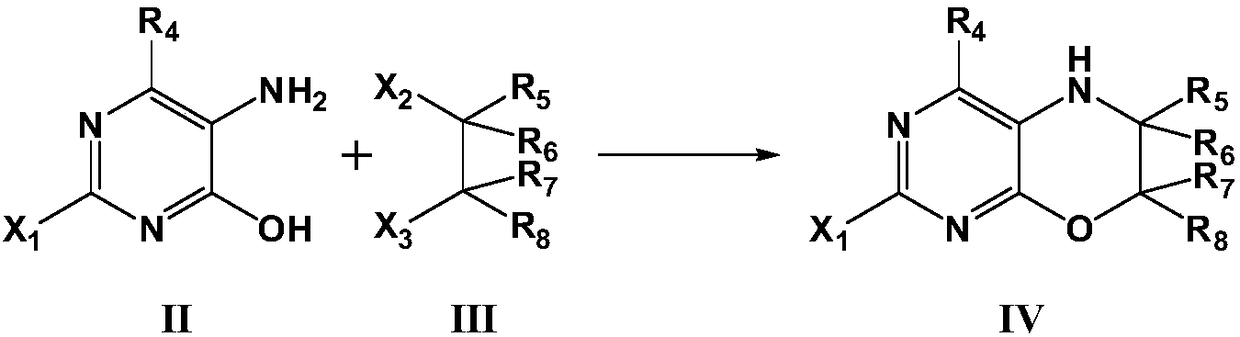
[0088] Step 1. Synthesis of 2-chloro-6,7-dihydro-5H-pyrimidine[4,5-b][1,4] Zinc
[0089]Add 60.0 mmol of 1,2-dibromoethane, 60.0 mmol of sodium hydroxide, and 0.75 g of benzyltriethylammonium chloride to a solution of 15.0 mmol of 5-amino-2-chloropyrimidin-4-ol in 200 mL of dichloromethane , and stirred at room temperature for 20 hours. After the reaction, the reaction mixture was poured into 300 mL of water, then extracted with ethyl acetate, the organic layer was washed with brine and water, and dried with anhydrous sodium sulfate to obtain 2-chloro-6,7-dihydro-5H-pyrimidine[ 4,5-b][1,4] 2.0 g of oxazine, yield 78.2%. LC-MS: m / z172.0(M+1) + .
[0090] Step 2. Synthesis of 2-(thiophen-3-yl)-6,7-dihydro-5H-pyrimidine[4,5-b][1,4] Zinc
[0091] Under nitrogen protection, 2-chloro-6,7-dihydro-5H-pyrimidine[4...
Embodiment 2
[0096] Embodiment 2: synthetic compound 1-2
[0097] 4-(3-(4-Methyl-2-(thiophen-3-yl)-6,7-dihydro-5H-pyrimidine[4,5-b][1,4] Azin-5-yl)propyl)-2-cyanopyridine
[0098]
[0099] According to the method of Example 1, the difference is that 4-methyl-5-amino-2-chloropyrimidin-4-ol is used instead of 5-amino-2-chloropyrimidin-4-ol, and 4-(3-bromopropane Base)-2-cyanopyridine in place of 4-(bromomethyl)-2-methoxypyridine to give 4-(3-(4-methyl-2-(thiophen-3-yl)-6,7- Dihydro-5H-pyrimidine[4,5-b][1,4] Oxyzin-5-yl)propyl)-2-cyanopyridine, the total yield of three steps is 51.3%.
[0100] LC-MS: m / z 378.1 (M+1) + .
[0101] Proton spectrum (400MHz, DMSO) δ8.84(d,1H),8.24(s,1H),8.01(d,1H),7.92(s,1H),7.78(d,1H),7.04(d,1H) , 4.25(t,2H), 3.55(t,2H), 3.47(t,2H), 2.65(t,2H), 2.31(s,3H), 2.02(m,2H).
Embodiment 3
[0102] Embodiment 3: synthetic compound 1-3
[0103] 2-(5-phenylfuryl-3-yl)-5-(3-(pyridin-4-yl)propyl)-6,7-dihydro-5H-pyrimidine[4,5-b][1 ,4] Zinc
[0104]
[0105] According to the method of Example 1, the difference is that 5-phenylfuran-3-boronic acid is used instead of thiophene-3-boronic acid, and 4-(3-bromopropyl)-pyridine is used instead of 4-(bromomethyl)-2- Methoxypyridine to give 2-(5-phenylfuryl-3-yl)-5-(3-(pyridin-4-yl)propyl)-6,7-dihydro-5H-pyrimidin[4, 5-b][1,4] oxazine, the three-step total yield is 49.1%.
[0106] LC-MS: m / z 399.1 (M+1) + .
[0107] Hydrogen spectrum (400MHz, DMSO) δ8.65(d,2H),8.24(s,1H),8.14(d,2H),7.61(t,2H),7.51(s,1H),7.40(t,1H) , 7.24(d,2H), 6.31(s,1H), 4.26(t,2H), 3.54(t,2H), 3.48(t,2H), 2.65(t,2H), 2.02(m,2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com