Synthetic method of D-heterocyclic amino acid, kit and application
A technology of a heterocyclic amino acid and a synthesis method, applied in the direction of fermentation, etc., can solve the problems of long synthesis route, long synthesis route, environmental pollution, etc., and achieve the effects of mild reaction conditions, stable process conditions, and high chiral purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
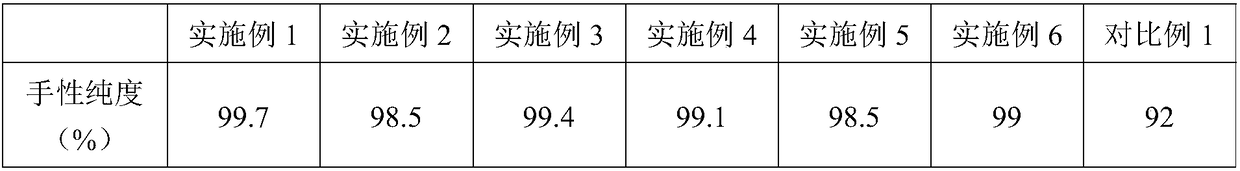
[0022] Based on the above research results, in a typical embodiment of the present application, a synthetic method of a D-heterocyclic amino acid is provided, the synthetic method comprising: using the dehydrogenation of diaminopimelic acid shown in SEQ ID NO:1 The enzyme converts D-heterocyclic ketoacids into D-heterocyclic amino acids. Using the diaminopimelate dehydrogenase shown in SEQ ID NO: 1 can achieve higher conversion rate and selectivity in the synthesis of D-heterocyclic amino acids.
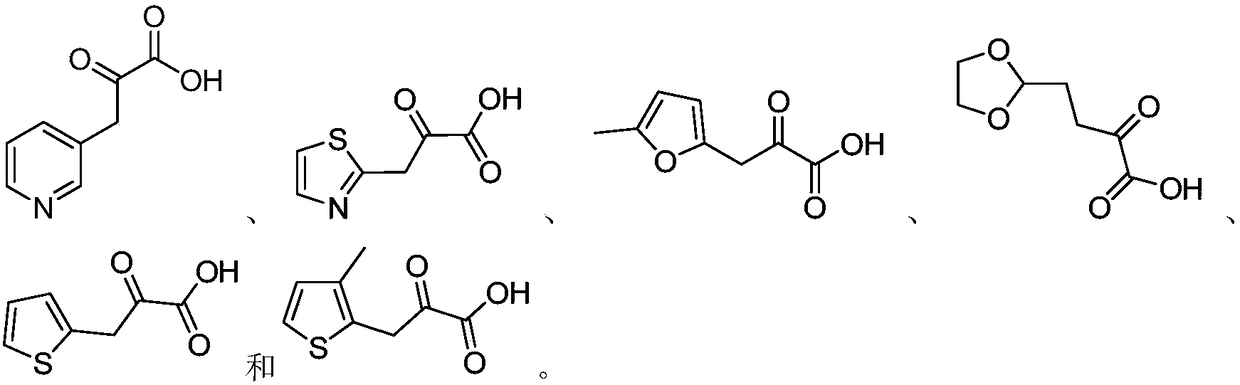
[0023] By screening a variety of existing D-heterocyclic ketoacid compounds, the inventors found that when any one of the following compounds is selected: , the synthesis with the above-mentioned diaminopimelate dehydrogenase has the advantage of high chiral purity.
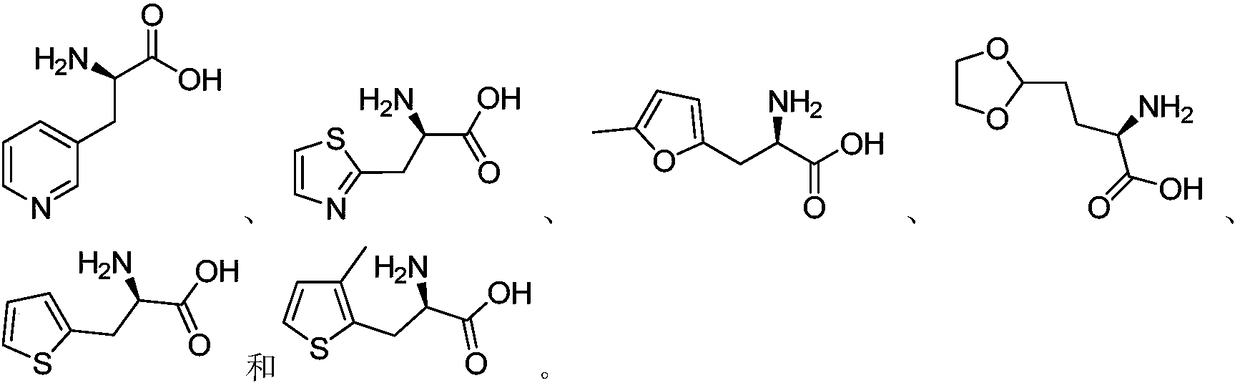
[0024] Correspondingly, when the above-mentioned D-heterocyclic ketoacid compounds are used as raw materials, the obtained D-heterocyclic amino acid is selected from any one of the following:
[0025] The above...
Embodiment 1
[0039] (1) Feeding: Add 10g of the main raw material into a 2L four-necked round bottom bottle 250ml Tris-HCl solution (100mmol / L, pH=9.0), the raw materials are evenly dispersed in the Tris-HCl salt buffer; use 10M, NaOH to adjust the pH to 8.5, then add 16g of ammonium formate, 0.3g of β-NAD + , and adjust the pH to 8.5 with 10M, NaOH. Finally with the diaminopimelate dehydrogenase of 80g, the formate dehydrogenase of 20g (cycle H + ) into the above reaction system, and adjust the pH to 8.5.
[0040] (2) Reaction: heat up to 30° C. for reaction.
[0041] (3) Post-treatment: track the system after 16 hours of reaction, detect the completion of the reaction of the raw materials, cool down to room temperature, slowly add concentrated hydrochloric acid dropwise to the four-neck flask to make the pH value of the mixed system after the reaction reach 1 to terminate the reaction, and pass the system over diatomaceous earth , the filtrate was adjusted to pH 8.0 with 30% NaOH, ex...
Embodiment 2
[0045] (1) Feeding: Add 10g of the main raw material into a 2L four-necked round bottom bottle 250ml Tris-HCl solution (100mmol / L, pH=9.0), the raw material is evenly dispersed in the Tris-HCl salt buffer; use 10M, NaOH to adjust the pH to 9.0, add 16.2g ammonium formate, 0.3gβ-NAD in turn + , and adjust the pH to 9.0 with 10M, NaOH. Finally, 40 g of diaminopimelate dehydrogenase and 20 g of formate dehydrogenase were added to the above reaction system, and the pH was adjusted to 9.0.
[0046] (2) Reaction: heat up to 40° C. for reaction.
[0047] (3) Post-treatment: track the system after reacting for 16 hours, detect the completion of the reaction of raw materials, drop to room temperature, slowly add concentrated hydrochloric acid dropwise to it under stirring to make the pH value of the mixed system after reaction to below 1; pass the acid-adjusted system over 1 ~2cm diatomite pad, wash the filter cake with 200ml purified water twice to obtain an aqueous solution, adjus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com