Carbon-based compounded ferric cyanamide material and preparation method thereof
A composite ferric cyanamide and carbon-based technology, which is applied in the direction of electrochemical generators, electrical components, battery electrodes, etc., can solve the problems of difficult to obtain composite structures, harsh synthesis and preparation conditions, and limited application of materials, so as to increase the magnification and cycle performance, reduce production cost, and solve the effect of volume expansion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1) Fully grind the analytically pure ferric ammonium oxalate and urea in a glass mortar to form a mixture A, wherein 1.5 g of ferric ammonium oxalate and 1.5 g of urea;
[0048] 2) Transfer A from the mortar to a quartz crucible, place the quartz crucible in a tube furnace, and raise the temperature to 150°C at a rate of 20°C / min under an argon atmosphere, and keep it warm for 1h, and then continue to heat up for 3 The heating rate of °C / min was continued to raise the temperature to 570 °C, and the temperature was kept for 1 h, and the obtained product was marked as B.
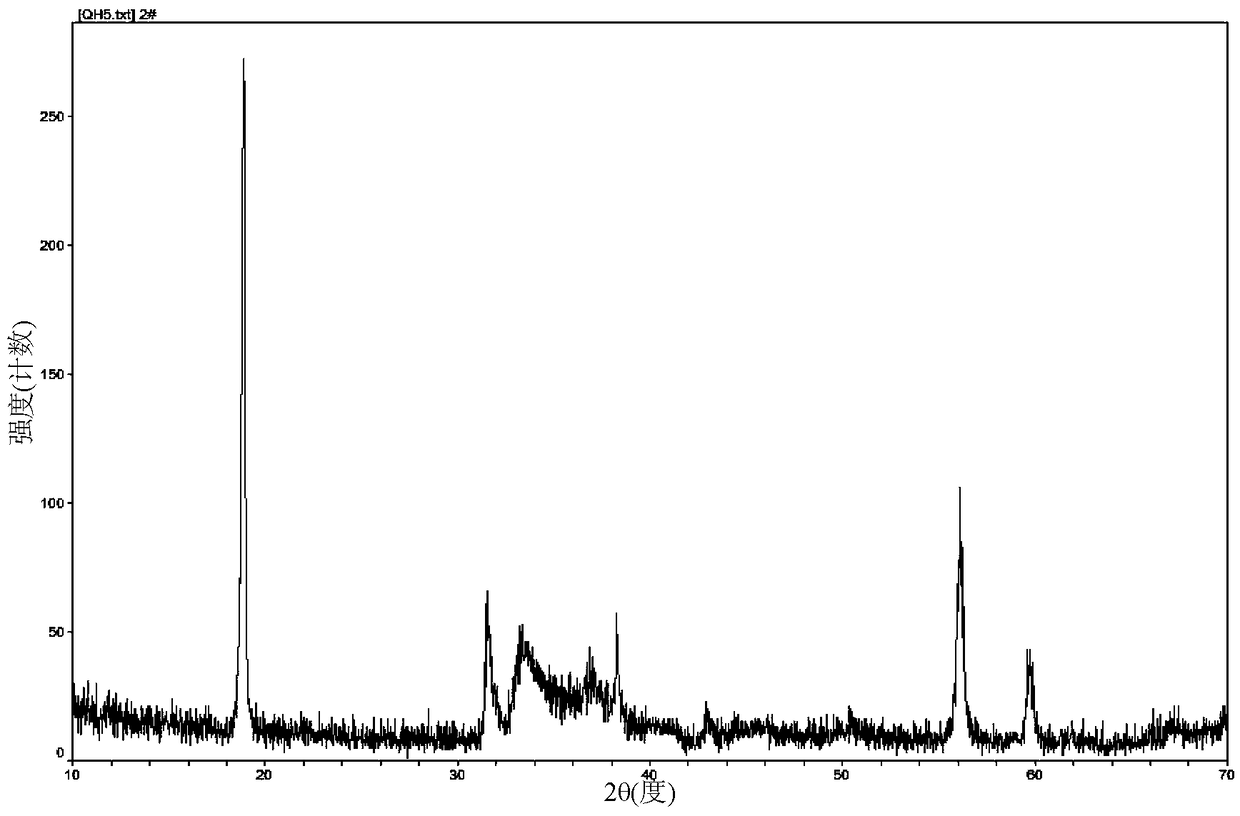
[0049] Adopt Japanese science D / max2000PCX-ray diffractometer to analyze product B, the XRD of gained product sees figure 1 . from figure 1 It can be seen that the product is a pure phase of FeCN 2 Structure, good crystallinity. Product B was observed under a scanning electron microscope and a transmission electron microscope, from Figure 2a and Figure 2b It can be seen that the product presents...
Embodiment 2
[0052] 1) Fully grind analytically pure ferric nitrate and melamine in a glass mortar to form mixture A, wherein 1 g of ferric nitrate and 1.67 g of melamine;
[0053] 2) Transfer A from the mortar to a quartz crucible, place the quartz crucible in a tube furnace, and raise the temperature to 160 °C at a rate of 30 °C / min under an argon atmosphere, keep it warm for 5 min, and then continue to heat it up for 5 min. The heating rate of °C / min was continued to raise the temperature to 600 °C, and the temperature was kept for 30 min, and the obtained product was marked as B.
[0054] Adopt Japanese science D / max2000PCX-ray diffractometer to analyze product B, the XRD of gained product sees image 3 . from image 3 It can be seen that the product is still pure phase FeCN 2 structure, but the crystallinity is weak, which may be related to the small particle size and the presence of a coating structure. The product B was observed under a scanning electron microscope and a transmi...
Embodiment 3
[0056] 1) Fully grind analytically pure ferrous oxalate and cyanamide in a glass mortar to form a mixture A, wherein 2 g of ferric nitrate and 0.4 g of cyanamide;
[0057] 2) Transfer A from the mortar to a quartz crucible, place the quartz crucible in a tube furnace, and raise the temperature to 160 °C at a rate of 30 °C / min under an argon atmosphere, keep it warm for 5 min, and then continue to heat it for 30 °C The heating rate of °C / min was continued to raise the temperature to 850 °C, and the temperature was kept for 1 min, and the obtained product was marked as B.
[0058] Adopt Japanese science D / max2000PCX-ray diffractometer to analyze product B, the XRD of gained product sees Figure 5a . from Figure 5a It can be seen that the product is still pure phase FeCN 2 Structure, good crystallinity, high purity. The product B was observed under a scanning electron microscope, from Figure 5b and Figure 5c It can be seen that the product presents a stacked polyhedral s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com