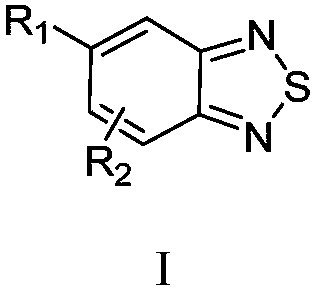
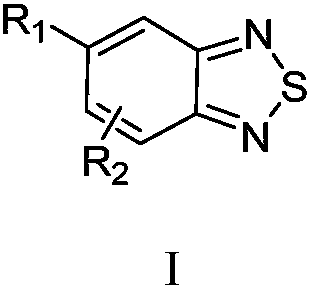
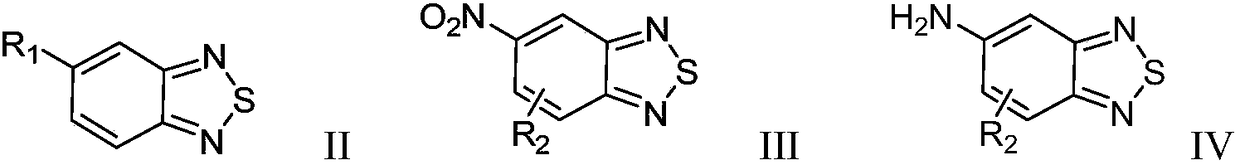
Benzothiadiazole compound and preparation method and use thereof
A technology of benzothiadiazoles and compounds, which is applied in the field of benzothiadiazoles, their preparation and application, and can solve problems such as the unclear mechanism of tumor action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Embodiment 1 (taking compound 6 synthesis as example):
[0113]
[0114] Reagents and conditions: a) p-toluenesulfonyl chloride, pyridine; b) fuming nitric acid, acetic acid; c) concentrated sulfuric acid; d) thionyl chloride, toluene; e) tert-butylacetylene, bistriphenylphosphine dichloride Palladium, cuprous iodide, triethylamine; f) iron powder, ammonium chloride, ethanol, water.
[0115] Add the pyridine solution (20mL) of p-toluenesulfonyl chloride (10.19g, 53.4mmol) to the pyridine solution (20mL) of 4-bromo-o-phenylenediamine (5g, 26.7mmol). Reflux and stir in the pot, react for 18h, after the thin layer chromatography monitors that the reaction is complete, place the reaction system at room temperature to cool, and slowly pour it into a mixed solution (250mL) of ice water:hydrochloric acid (4:1), there is The solid was precipitated, filtered with suction, and the filter cake was washed with distilled water to obtain a brown solid product, which was dried for...
Embodiment 2
[0125]
[0126] Reagents and conditions: a) trimethylethynyl silicon, bistriphenylphosphine palladium dichloride, cuprous iodide, triethylamine; b) tetrabutylammonium fluoride, tetrahydrofuran; c) iron powder, chloride Ammonium, ethanol, water; d) palladium carbon, hydrogen.
[0127] Will contain compound 4 (400mg, 1.54mmol), PdCl 2 (PPh 3 ) 2 (54mg, 0.07mmol), CuI (14.6mg, 0.07mmol) and Et 3 The mixture of N (4mL) was placed in an oil bath at 50°C, trimethylethynyl silicon (300mg, 3.08mmol) was added dropwise, and reacted for about 2h. After monitoring the completion of the reaction, diluted with ethyl acetate and water, extracted, and combined phase, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography (30:1 petroleum ether / ethyl acetate) to obtain compound 7 (300 mg, 70.8%).
[0128] Under ice bath condition, tetrabutylammonium fluoride (1.6mL, 5.84mmol) was added dropwise to a THF (3mL) solution of compound 7 (250mg, 0.903mmol). ...
Embodiment 3
[0132]
[0133] Reagents and conditions: a) benzyl azide, cuprous iodide, glycerin; b) iron powder, ammonium chloride, ethanol, water.
[0134] A mixture of compound 8 (20mg, 0.098mmol), benzyl azide (14mg, 0.098mmol), CuI (0.92mg, 0.0048mmol) and glycerol (0.5mL) was placed in a 40°C oil bath for overnight reaction. After the reaction was monitored by thin-layer chromatography, it was quenched with water, extracted with ethyl acetate, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography (6:1 petroleum ether / ethyl acetate) to obtain compound 11 (20mg, yield 62.5%). 1 H NMR (400MHz, CDCl 3 )δ:8.57(s,1H),8.46(s,1H),7.70(s,1H),7.45-7.32(m,5H),5.63(s,2H).
[0135] Will contain compound 11 (20mg, 0.059mmol) and NH 4 The solution of Cl (12.5mg, 0.237mmol) in ethanol and water (2:1, 1mL) was placed in an oil bath at 90°C for reflux reaction for 30min, then iron powder (13.27mg, 0.237mmol) was added, and the reaction was continued for 1h under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com