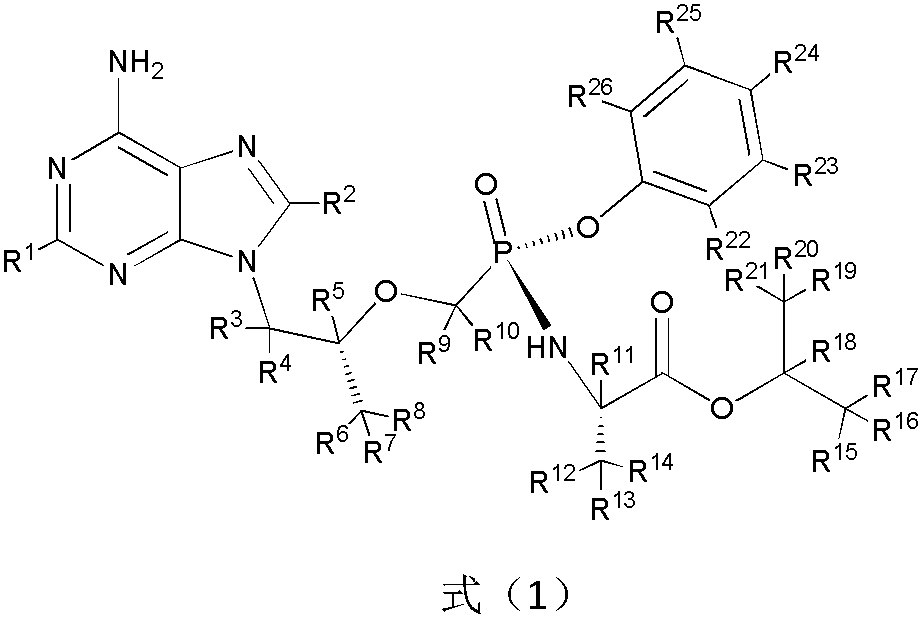
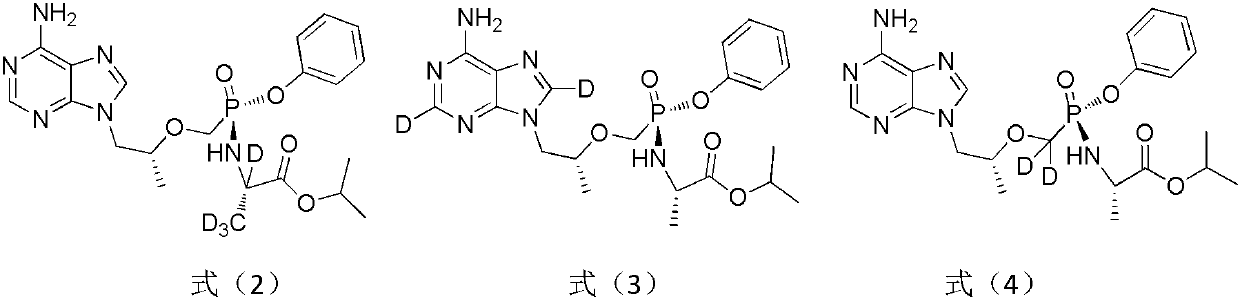
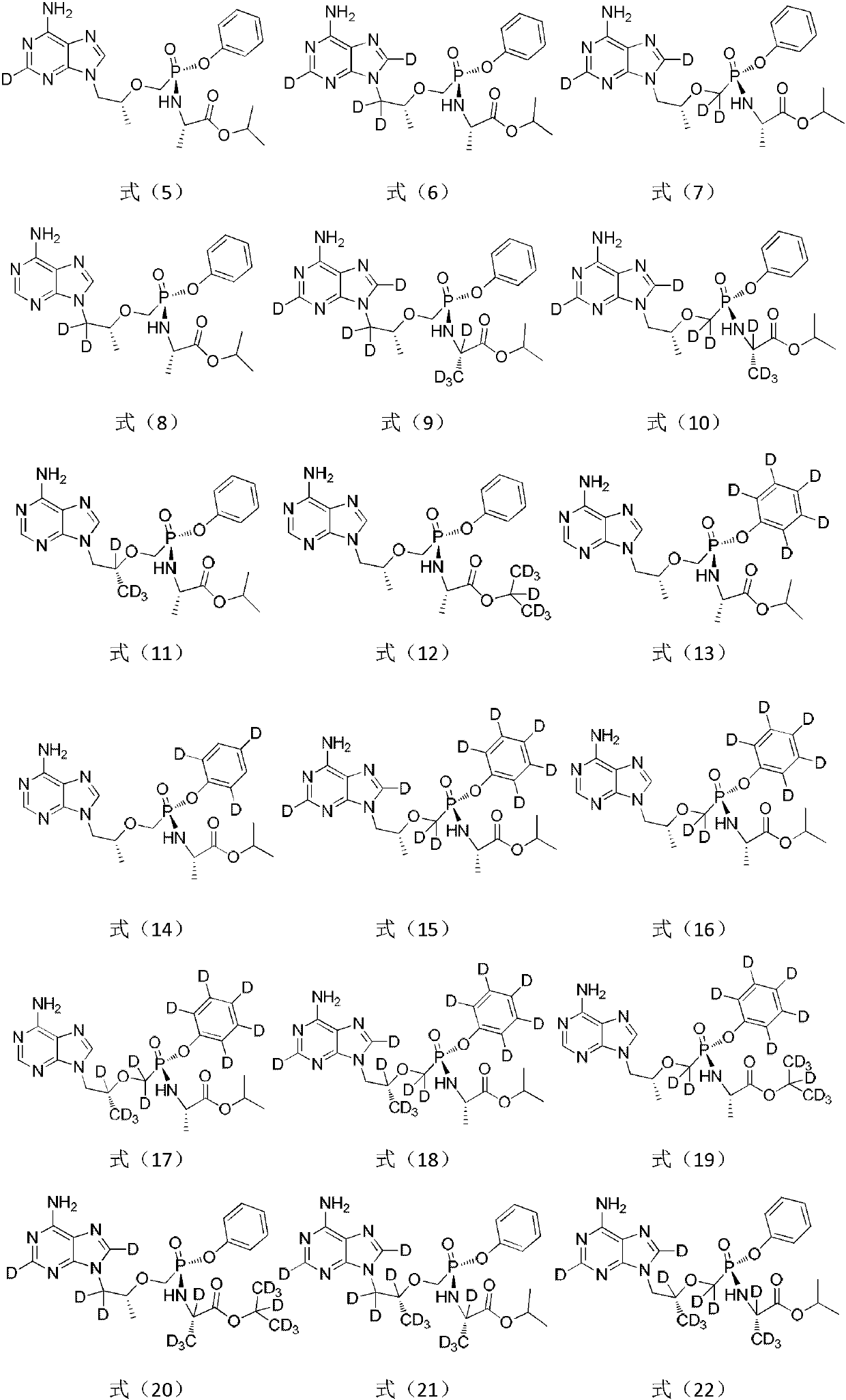
A substituted adenine compound and its pharmaceutical composition
A compound and pharmaceutical technology, applied in the field of medicine, can solve problems such as toxic side effects and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 Preparation of 9-{(R)-2-[((S)-{[(S)-1-(isopropoxycarbonyl) ethyl]amino}-2,4,6-d3-phenoxyphos Acyl) methoxyl group] propyl group} adenine, namely compound T-1, molecular formula is as follows:
[0050]
[0051] Synthesize using the following route:
[0052]
[0053]
[0054] Step 1 Synthesis of (R)-9-(2-hydroxypropyl)adenine (Compound 1).
[0055] Add adenine (4.0g, 29.6mmol) and (R)-propylene carbonate (3.45g, 33.8mmol) into the reaction flask, add 4.5ml DMF to dissolve, heat to 130°C to react overnight, and spot the plate to detect that the reaction is complete. Cool down to 100°C, add 14ml of toluene and 0.47g of methanesulfonic acid (keep the internal temperature at 100-110°C), then add 11ml of toluene to obtain a homogeneous suspension, gradually cool down to room temperature, then cool down to 0°C for 1 hour , filtered white solid, and dried in vacuo to obtain 5.77 g of product, yield 100%. LC-MS(APCI): m / z=194.3(M+1)+ .
[0056] Step 2 Synth...
Embodiment 2
[0070] Example 2 Preparation of 9-{(R)-2-[((S)-{[(S)-1-(d7-isopropoxycarbonyl)ethyl]amino}phenoxyphosphoryl)methoxy]propane Base} adenine, namely compound T-2, its molecular formula is:
[0071]
[0072] Adopt the following synthetic route:
[0073]
[0074]
[0075] Step 1 (R)-Synthesis of 9-[2-(phenoxyphosphorylmethoxy)propyl]adenine (Compound 8).
[0076] Add compound 4 (2.4g, 8.36mmol), phenol (1.62g, 16.72mmol) and 6.5ml of NMP into the reaction flask, heat to 85°C, add triethylamine (1.04g, 10.3mmol), heat up to 100°C, add Dicyclohexylcarbodiimide (2.81g, 13.63mmol) was raised to 120°C and stirred for 16 hours. Spot the plate to detect the disappearance of the raw materials and cool down to 45°C, add 4.8ml of water, cool down to room temperature, filter to remove insoluble matter, wash the filter cake with 2.5ml of water, concentrate the filtrate, add 4ml of water, adjust the pH to 11 with NaOH, and extract with chloroform for 3 -4 times, the aqueous phase wa...
Embodiment 3
[0083] Example 3 Preparation of 9-{(R)-2-[((S)-{[(S)-1-(isopropoxycarbonyl)ethyl]amino}-d5-phenoxyphosphoryl)methoxy] Propyl}adenine, namely compound T-3, its molecular formula is:
[0084]
[0085] Adopt following synthetic route:
[0086]
[0087] Step 1 Synthesis of 2,3,4,5,6-d5-phenol (Compound 11).
[0088] Add phenol (2.0g, 21.25mmol), 5% Pt / C (0.4g, 20wt%) and 34ml of heavy water in the reaction flask, replace the hydrogen 3-4 times, react at room temperature for 24 hours, remove the catalyst by filtration, and use the After washing with dichloromethane, the combined organic phases were washed with saturated brine, dried over anhydrous sodium sulfate, filtered and concentrated, and then purified by column chromatography to obtain 1.6 g of compound 11 with a yield of 80%. LC-MS(APCI): m / z=100.4(M+1) + .
[0089] Step 2 Synthesis of (R)-9-[2-(d5-phenoxyphosphorylmethoxy)propyl]adenine (compound 12).
[0090] Add compound 4 (2.4g, 8.36mmol), compound 11 (1.66g, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com