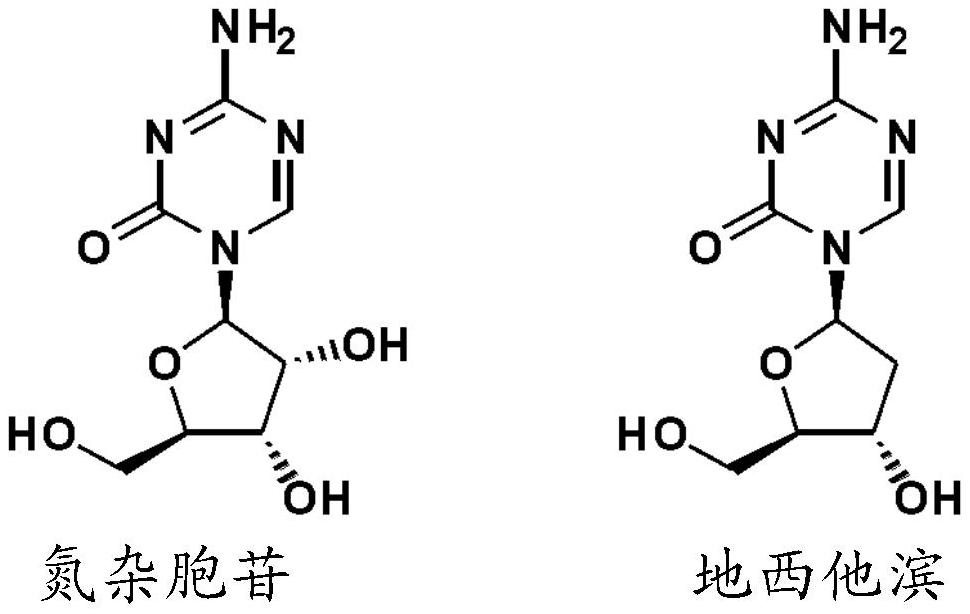
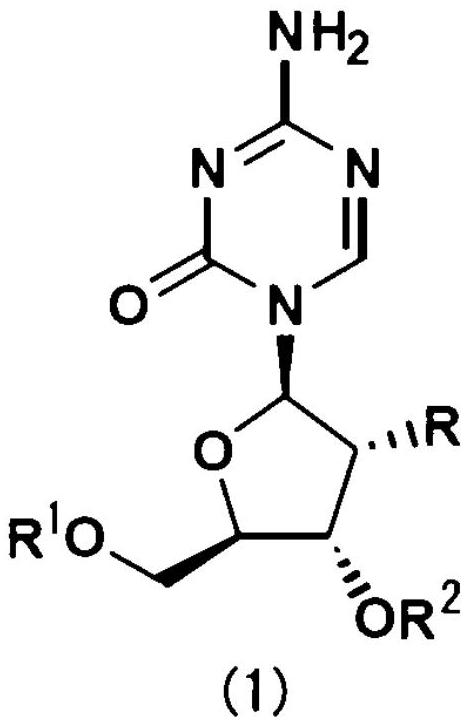
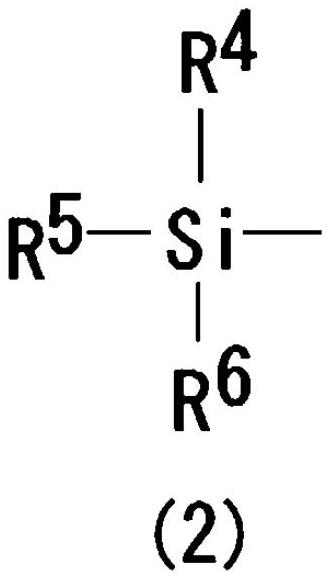
The sugar moiety silyl ether derivatives of 5-azacytidine
A silyl group and azacytidine technology, applied in sugar derivatives, drug combinations, extracellular fluid diseases, etc., can solve the problems of undisclosed and disclosed stability and reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0075] The preparation method of compound (1) of the present invention
[0076] For example, the compound (1) of the present invention can be prepared according to the following method or other similar methods (for example, the silyletherification method disclosed in Corey, E.J.et al., J.Am.Chem.Soc., 94, 6190 , 1972; Morita, T.etal., Tetrahedron Lett., 21, 835, 1980; Y.Kita, et al., Tetrahedron Lett., 4311, 1979etc.; As a review, refer to Lalonde, M., Chan, T.H., Synthesis, 817-845, 1985 etc.).
[0077] Compound (1) or a salt thereof can be produced according to a conventional method or a method analogous thereto. For example, commercially available 5-azacytidine or 2'-deoxy-5-azacytidine is reacted with a silyl halide compound in a suitable solvent in the presence of a base. A sugar moiety silyl ether derivative of 5-azacytidine can be obtained as the target compound.
[0078] Silyl halide compound
[0079] The kind of the silyl halide compound is not particularly lim...
Embodiment 1
[0110] Synthesis of 5'-(trisubstituted)silyloxy-5-azacytidines (5'-(trisubstituted)silyloxy-5-azacytidines, 1a)
[0111]
[0112]Add imidazole (1.5 mM) to a suspension of 5-azacytidine (I) (1 mM) in dry N,N-dimethylformamide (3 mL), and then add the corresponding silyl group dropwise under ice-bath Chlorine (1.2mM) for about 10 minutes. It is then stirred for about 1 to 17 hours until gradually warming to room temperature and starting material disappears. The reaction solution was poured into 50 mL of a mixture of ethyl acetate / saturated brine (2:1) and extracted with ethyl acetate. The extract was washed twice with saturated brine (10 mL), and dried over anhydrous sodium sulfate. The extract after removing the insoluble matter was concentrated to dryness under reduced pressure. The obtained oily residue was separated and purified with a silica gel column (YamazenSmart Flash MS system), thereby obtaining a 5'-silyl ether derivative of the target compound 5-azacytidine (c...
Embodiment 2
[0114] Synthesis of 3',5'-di(trisubstituted)silyloxy-5-azacytidines (3',5'-di(trisubstituted)silyloxy-5-azacytidines, 1b)
[0115]
[0116] To a suspension of 5-azacytidine (I) (1 mM) in anhydrous N,N-dimethylformamide (3 mL) was added imidazole (2 mM), followed by the corresponding silyl chloride dropwise in an ice bath (1.5mM) for about 10 minutes. The mixture was stirred for several hours until gradually warming to room temperature and starting material disappeared. The reaction solution was poured into 50 mL of a mixture of ethyl acetate / saturated brine (2:1) and extracted with ethyl acetate. The extract was washed twice with saturated brine (10 mL), and dried over anhydrous sodium sulfate. The extract after removing the insoluble matter was concentrated to dryness under reduced pressure. The obtained oily residue was separated and purified with a silica gel column (YamazenSmart Flash MS system), thereby obtaining the 3',5'-disilyl ether derivative of the target comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com