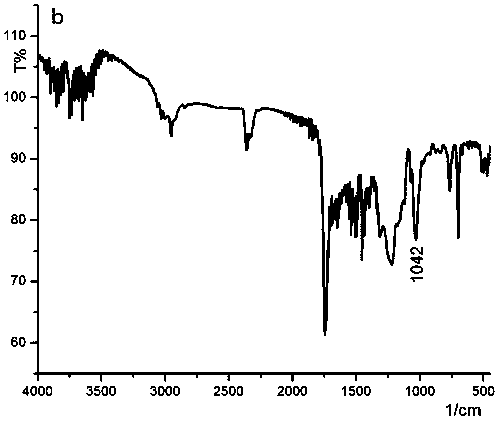
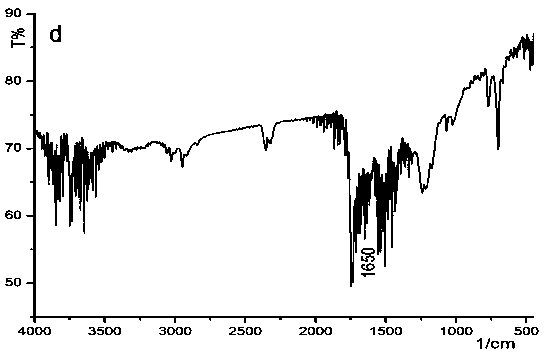
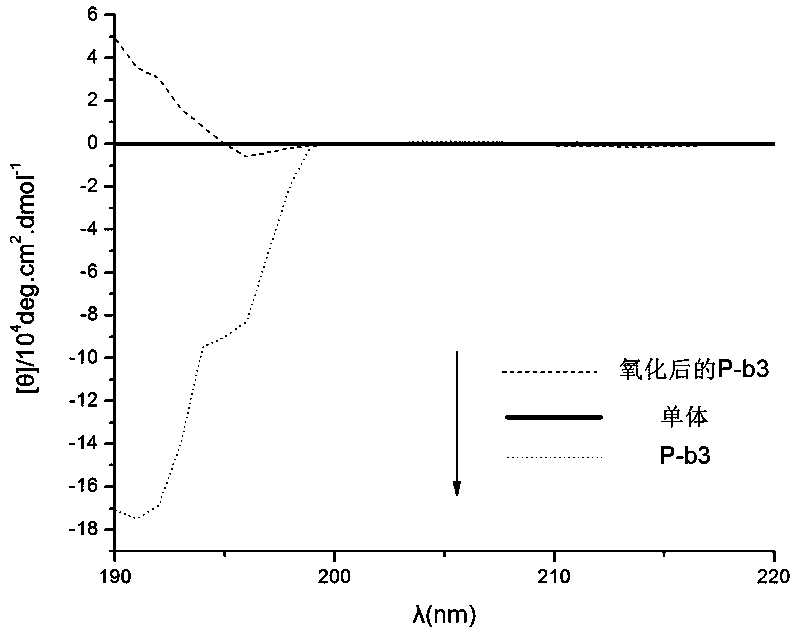
A kind of isonitrile helical polymer containing thioether side chain with reversed oxidized helical conformation and preparation method thereof
A helical conformation and thioether-containing technology, which is applied in the field of isonitrile helical polymers and their preparation, can solve problems such as the application of complexes that cannot be helical, and achieve stimulating effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Preparation of S-benzyl-L-cysteine:
[0037]Add 2.42 g (0.02 mol) of L-cysteine to a 250 ml four-neck flask, add 37.5 ml of 2.13 mol / L NaOH solution to dissolve it, control the temperature to 45 ° C, and then dropwise add 2.4 ml (0.02 mol ) of benzyl bromide, stirred thoroughly for 30 min, and adjusted the pH value with diluent of acetic acid made of 10 ml acetic acid and 40 ml distilled water until the pH was 5.4, at which time a large amount of white precipitates precipitated out. Stir well and filter with suction to obtain a white solid, which is then washed with distilled water, dried in a vacuum oven, and weighed to obtain 4.14 g of white solid powder with a yield of 97.6%, mp: 215.5-216.4 °C. IR (KBr, cm -1 ): 1619, 1568, 1493 (C=C on the benzene ring); 1394 (C-N); 698,768 (Ar-H).
[0038] The reaction equation of this process is as follows;
[0039]
[0040] (2) Preparation of S-benzyl-L-cysteine methyl ester:
[0041] In a 100 ml four-neck flask, ...
Embodiment 2
[0063] The specific implementation content in the second embodiment is the same as that of the first, but the difference is that the reaction temperature of the step (1) is 35°C; the molar ratio of formic acid and acetic anhydride in the formic acetic anhydride in the step (3) is 3; in the step (4) The molar ratio of dichloromethane and triethylamine is 2, and the molar ratio of triphosgene and dichloromethane is 0.5.
Embodiment 3
[0065] The specific implementation content in the third embodiment is the same as the first one, but the difference is that the reaction temperature of the step (1) is 65°C; the molar ratio of formic acid and acetic anhydride in the formic acetic anhydride in the step (3) is 10; in the step (4) The molar ratio of dichloromethane to triethylamine is 5, and the molar ratio of triphosgene to dichloromethane is 2.
[0066] The properties and structural characteristics of the materials prepared in Example 2 and Example 3 are the same or similar to those in Example 1.
[0067]The polymer containing the thioether structure synthesized by the invention has a helical structure and has redox stimulus responsiveness. Because it contains S atoms, it can be oxidized by oxidants to achieve redox stimulation. Such polyisonitrile helical polymers containing thioether structures can be used in the fields of chiral recognition, chiral separation, chiral signal amplification, asymmetric catalys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com