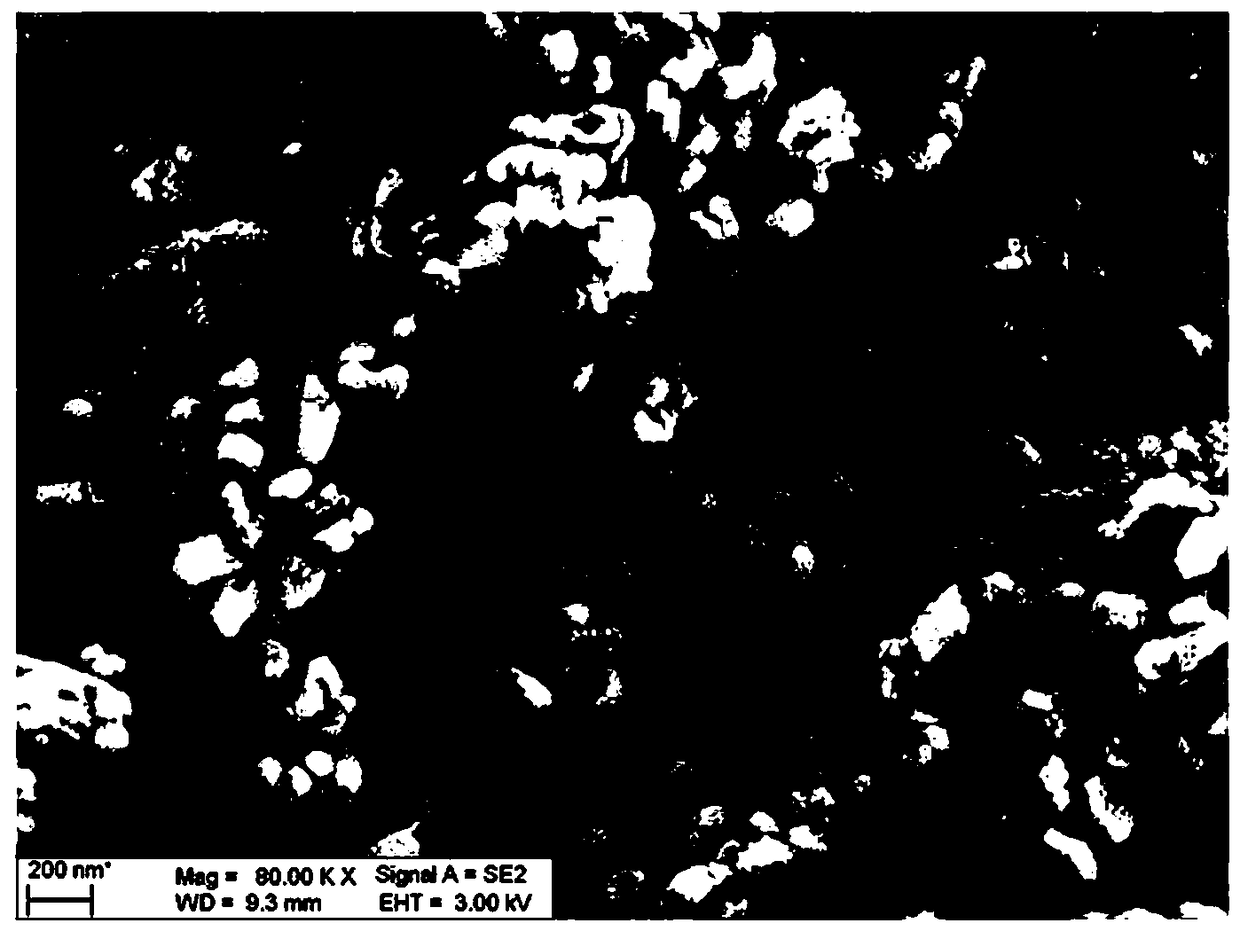
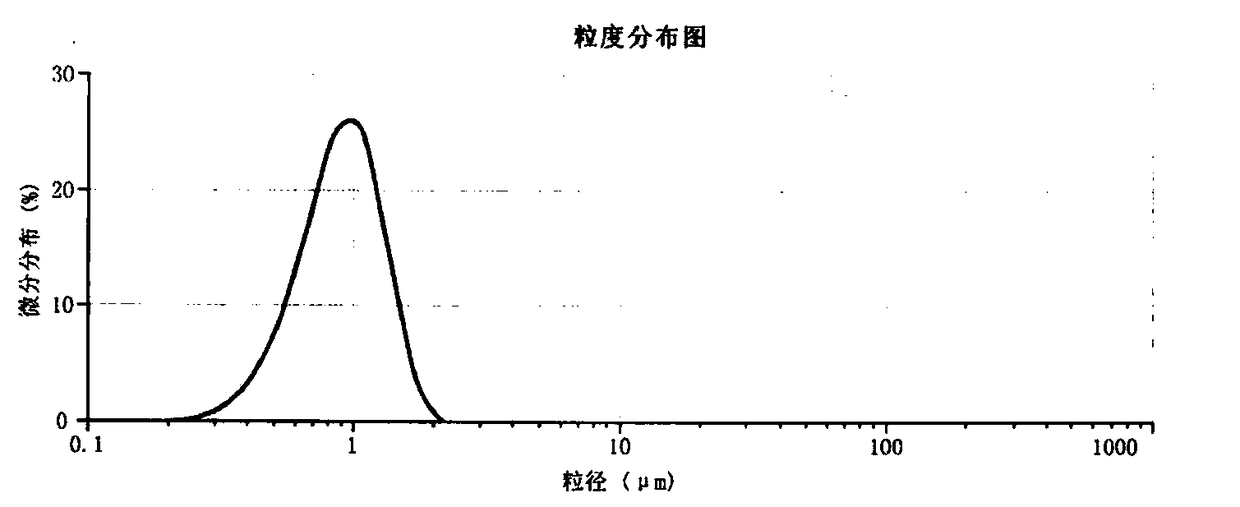
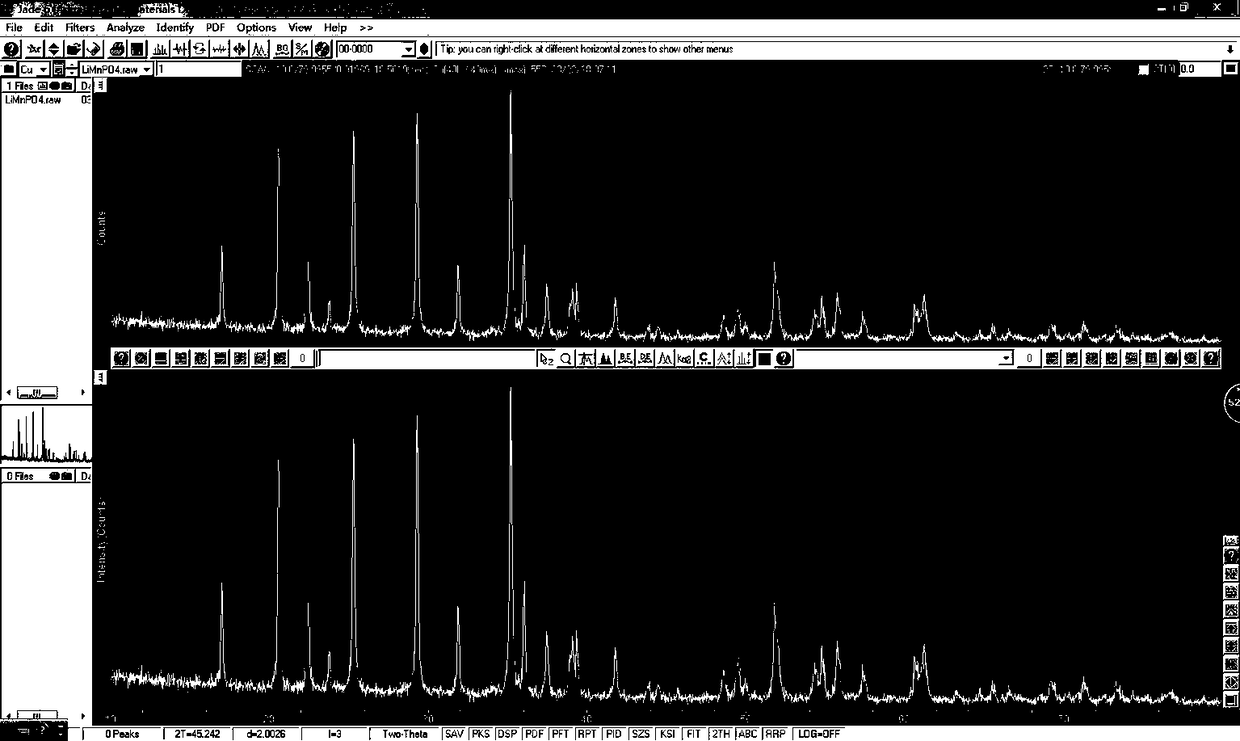
Preparation method of pollution-free low-cost lithium manganese ferric phosphate crystal material
A technology of lithium iron manganese phosphate and crystalline materials, applied in phosphorus compounds, inorganic chemistry, non-metallic elements, etc., can solve the problems of unsolved safety problems, limited energy density, and difficulty in charging and discharging. The effect of low synthesis cost, increased synthesis cost, and improved overall level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation process of pollution-free and low-cost lithium manganese iron phosphate crystal material, comprising the following steps:
[0033] 1) Weigh iron powder, manganese powder and phosphoric acid according to the molar ratio of 0.3:0.7:1, and the diluted concentration of phosphoric acid is 40%;
[0034] 2) Put the substance weighed in step 1) into the acid-resistant reactor to control the temperature of the reaction system between 30°C, feed oxygen to start the pre-reaction, and the reaction time is 24 hours;
[0035] 3) Step 2) Add lithium hydroxide solution after the reaction, lithium hydroxide and phosphoric acid are mixed at a ratio of 1:1, after mixing evenly, the precursor is transferred into an autoclave, and the reaction is sealed. The temperature during the synthesis process is 120-260 ° C, high pressure The pressure of the kettle is 0.2-4.7MPa, the whole reaction process is continuously dispersed and stirred, the reaction time is controlled within 3-24...
Embodiment 2
[0039] A preparation process of pollution-free and low-cost lithium manganese iron phosphate crystal material, comprising the following steps:
[0040] 1) Weigh iron powder, manganese powder, and phosphoric acid according to the molar ratio of 0.5:0.5:1-1.2, and the diluted concentration of phosphoric acid is 35%;
[0041] 2) Put the substance weighed in step 1) into the acid-resistant reactor to control the temperature of the reaction system between 25 and 80°C, feed oxygen to start the pre-reaction, and the reaction time is 18 hours;
[0042] 3) Step 2) After the reaction, add lithium hydroxide solution, lithium hydroxide and phosphoric acid are mixed at a ratio of 1-1.2:1, after mixing evenly, the precursor is transferred into an autoclave, and the reaction is sealed. The temperature during the synthesis process is 120-260°C , the pressure of the autoclave is 0.2-4.7MPa, the whole reaction process is continuously dispersed and stirred, the reaction time is controlled within...
Embodiment 3
[0046] A preparation process of pollution-free and low-cost lithium manganese iron phosphate crystal material, comprising the following steps:
[0047] 1) Weigh iron powder, manganese powder, and phosphoric acid according to the molar ratio of 0.4:0.6:1-1.2, and the diluted concentration of phosphoric acid is 30%;
[0048] 2) Put the substance weighed in step 1) into the acid-resistant reactor to control the temperature of the reaction system between 25 and 80°C, feed oxygen to start the pre-reaction, and the reaction time is 18 hours;
[0049]3) Step 2) After the reaction, add lithium hydroxide solution, lithium hydroxide and phosphoric acid are mixed at a ratio of 1-1.2:1, after mixing evenly, the precursor is transferred into an autoclave, and the reaction is sealed. The temperature during the synthesis process is 120-260°C , the pressure of the autoclave is 0.2-4.7MPa, the whole reaction process is continuously dispersed and stirred, the reaction time is controlled within ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com