Preparation method for R-3-aminobutanol
A technology of aminobutanol and R-3-, which is applied in the field of preparation of chiral drug intermediate R-3-aminobutanol, can solve the problems of reduced yield, slow reaction speed and low yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
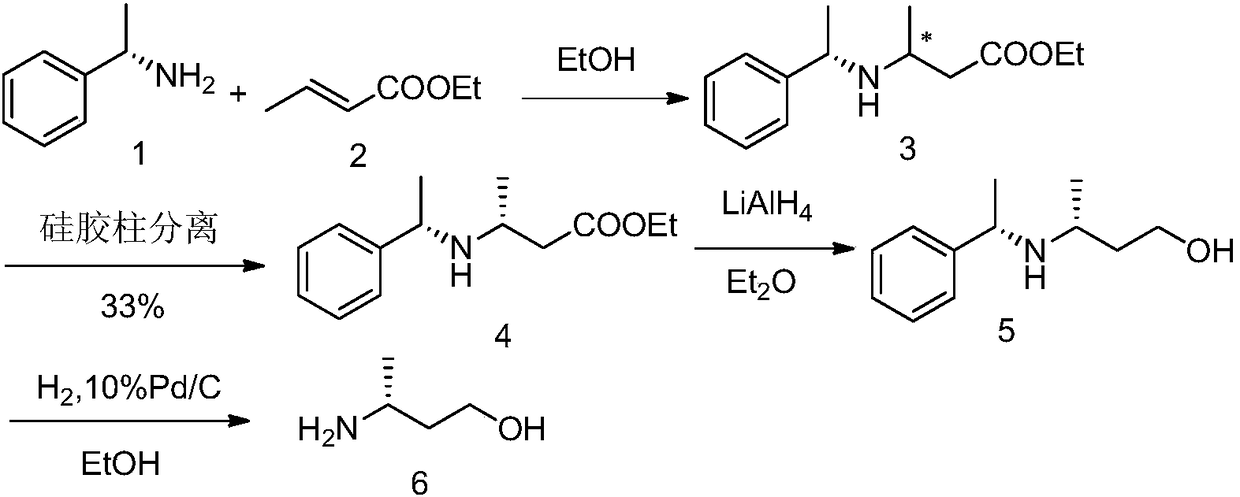
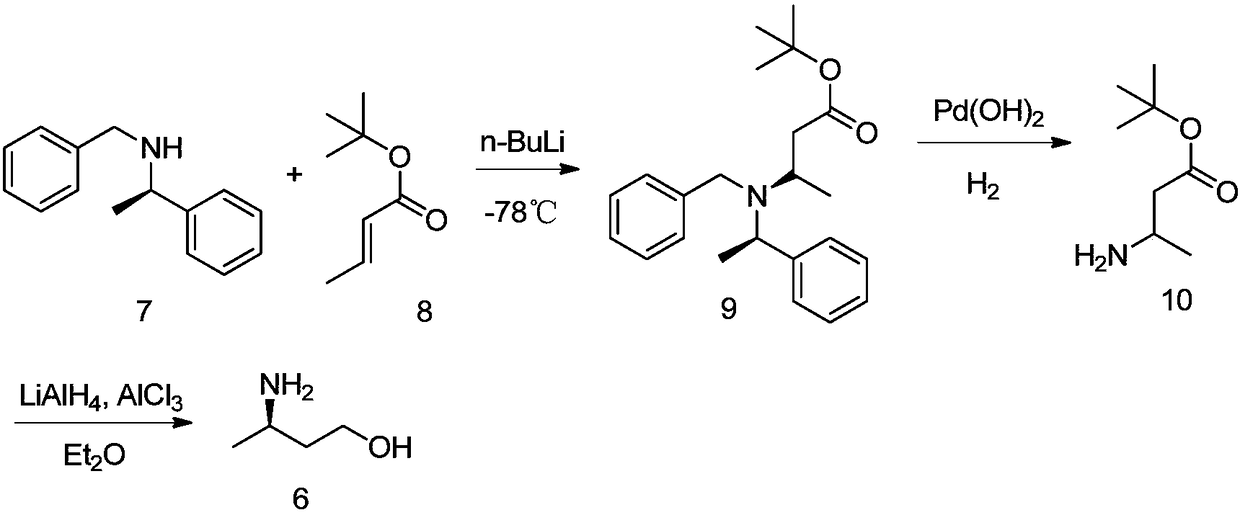
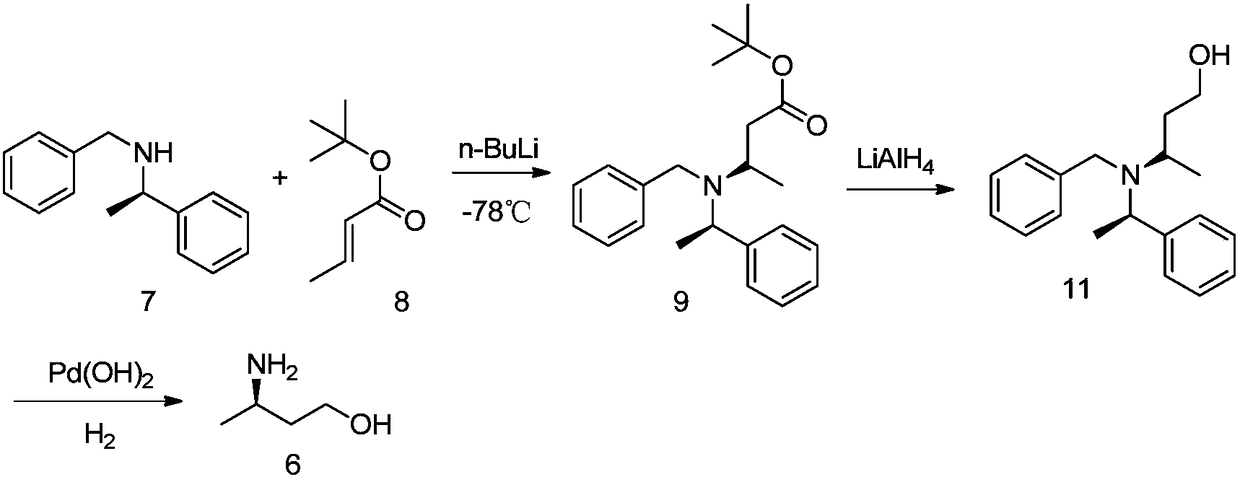
[0065] A kind of preparation method of R-3-aminobutanol of the present invention, as shown in following reaction formula:
[0066]
[0067] The reaction steps are:
[0068] (1) R-3-aminobutyric acid and di-tert-butyl carbonate, use water as a solvent, and protect the amino group to obtain N-Boc-(R)-3-aminobutyric acid;
[0069] (2) N-Boc-(R)-3-aminobutyric acid, using sodium borohydride as a reducing agent, Lewis acid as a catalyst, reduction to obtain N-Boc-(R)-3-aminobutanol;
[0070] (3) N-Boc-(R)-3-aminobutanol, under the condition of hydrochloric acid / methanol, remove the Boc protection to obtain (R)-3-aminobutanol.
[0071] For further understanding content of the present invention, each step is specifically described as follows:
[0072] In the reaction step (1) of the present invention, the molar ratio of R-3-aminobutyric acid to di-tert-butyl carbonate is usually 1:1-1:1.5; the solvent used for the reaction is water, and the reaction time is 2-6 hours;
[0073] ...
Embodiment 1
[0087] Embodiment 1: the preparation of N-Boc-(R)-3-aminobutyric acid
[0088] Add 103g of R-3-aminobutyric acid, 105g of sodium carbonate, and 218g of di-tert-butyl carbonate into a reaction bottle filled with 500mL of water, and react at 25°C for 2-6 hours. After the reaction is complete, adjust the pH to 3 with 2N hydrochloric acid. -4, the product was extracted twice with 500mL ethyl acetate, spin-dried to obtain N-Boc-(R)-3-aminobutyric acid (I), 190g of white solid, yield 93.5%, content 98% (HPLC method ).
[0089] H NMR (400MHz, DMSO-d6) δ12.111 (1H, s, COOH), 6.725-6.744 (1H, d, J=7.6Hz, NH), 3.745-3.815 (1H, m, CH), 2.180-2.422 (2H,m,CH 2 ),1.368(9H,s,3CH 3 ), 1.037 (3H, d, J=6.4Hz, CH 3 );
Embodiment 2
[0090] Embodiment 2: the preparation of N-Boc-(R)-3-aminobutanol
[0091] Take 100g of N-Boc-(R)-3-aminobutyric acid and 300mL of tetrahydrofuran into the reaction flask, add 20.6g of sodium borohydride in batches, cool down to -20°C, slowly drop in 100g of boron trifluoride ether, HPLC Check whether the reaction of raw materials is complete. After the reaction is complete, add methanol to quench the reaction, concentrate under reduced pressure to remove the solvent, add 500ml of ethyl acetate, filter to remove the solid, wash the filtrate with 100ml of saturated sodium bicarbonate, and concentrate the organic phase to obtain N-Boc-(R)-3-amino Butanol, white solid, 88g, yield 95%, content 99.0% (HPLC method).
[0092] H NMR (400MHz, DMSO-d6) 6.585 (1H, d, J = 8.4Hz, NH), 4.286-4.311 (1H, t, OH), 3.484-3.518 (1H, m, CH), 3.318-3.371 (2H ,m,CH2),1.379-1.519(2H,m,CH2),1.335(9H,s,3CH3,),0.965(3H,d,J=6.4Hz,CH3)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com