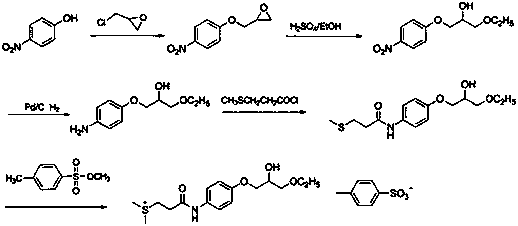
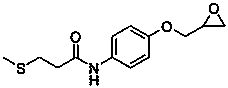
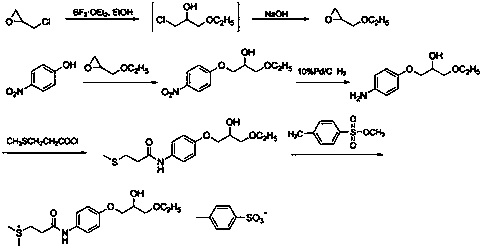
Preparation methods of suplatast tosilate and intermediates
A technology of sulfamilast and intermediates, which is applied in the field of preparation of sulfamilast and its intermediates, can solve the problems of difficulty in reaction monitoring and purification, poor production operability, and high safety risks, and avoid palladium-carbon catalytic hydrogenation reduction The steps of nitro group are beneficial to the production operation and the effect of shortening the reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of Intermediate 1
[0038] At 20~30°C, add 20.0 g (0.18 mol) of 4-aminophenol and 2-methyltetrahydrofuran (200 mL) into the reaction flask, add 30.8 g (0.55 mol) of crushed calcium oxide under stirring, Stir for 0.5~1.0 h, cool down to -10~5°C, control the temperature at -10~5°C, add 28.0 g (0.20 mol) of 3-methylthiopropionyl chloride dropwise, after 0.5~1 h dropwise, raise the temperature to 20~30 Stir at ℃ for 4~6 h, the raw material point disappeared in TLC, filter with suction, wash the filter cake with 100 mL of 2-methyltetrahydrofuran, collect the filtrate and wash with 200 mL of saturated sodium bicarbonate, separate the layers, and the organic layer (2-methyltetrahydrofuran Tetrahydrofuran) was dried with an appropriate amount of anhydrous magnesium sulfate, filtered, and the filtrate was distilled off under reduced pressure to remove the solvent to obtain 32.0 g of intermediate 1 with a yield of 83.0%, which was directly used in the next r...
Embodiment 2
[0040] Example 2 Preparation of Intermediate 2
[0041] At 20~30°C, add 10.0 g (47.33 mmol) of intermediate 1, 13.1 g of epichlorohydrin (141.99 mmol), and 0.1 g (1 mmol) of triethylamine into the reaction flask, stir and heat up to 60~70°C for reaction After 6~8 h, the point of raw material in TLC disappears, distill under reduced pressure, cool the residue to room temperature, add 200ml of acetone, 150ml of 5 mol / L KOH aqueous solution, stir at 20~30°C for 3 hours, keep the temperature below 30°C , evaporate the solvent under reduced pressure, cool to room temperature, extract with 3×150ml dichloromethane, wash with 3×150ml saturated brine, distill to dryness, add 250ml ethanol, 2.5g activated carbon, stir and reflux at 20~30°C for 3 hours for decolorization , filtered while hot, the filter cake was washed with a small amount of ethanol, the filtrates were combined, and about 150 ml of ethanol was evaporated by heating to obtain 9.6 g of intermediate 2, with a yield of 75.9%...
Embodiment 3
[0042] Example 3 Preparation of Intermediate 3
[0043] At 20~30°C, add 8.0 g (29.92 mmol) of intermediate 2, ethanol (160 mL), 0.4 g (1.14 mmol) of tin tetrachloride pentahydrate into the reaction flask, stir, heat to reflux for 2 h, and depressurize Ethanol was distilled off. The residue was cooled to room temperature, added 100 mL of dichloromethane, washed with 80 mL of 2mol / L HCl aqueous solution, and then washed with 2×40 mL of saturated brine, the organic phase was collected, spin-dried, and recrystallized by adding 80 mL of acetone to obtain 7.3 g of intermediate Body 3, yield 77.8%. 1 H-NMR (DMSO-d 6 )δ: 1.06~1.11(t, 3H, CH 3 ),2.06(s, 3H, S-CH 3 ),2.48~2.74(m, 4H, SCH 2 CH 2 ),3.40~3.47 (m, 4H,CH 2 OCH 2 ),3.81~3.89(m, 3H, CH, Ar-OCH 2), 4.00~5.00(d, 1H, OH), 6.81~6.84(d, 2H, Ar-H), 7.44~7.47 (d, 2H, Ar-H), 9.75 (s, 1H, CONH).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com