Rapid preparation method of chloroiridic acid
A rapid technology of chloroiridic acid, applied in the field of preparation of rare and precious metal chemicals, can solve problems such as being unsuitable for industrial production, small processing volume, complicated operation, etc., and achieve the effects of increasing market competitiveness, high conversion rate, and simple principle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
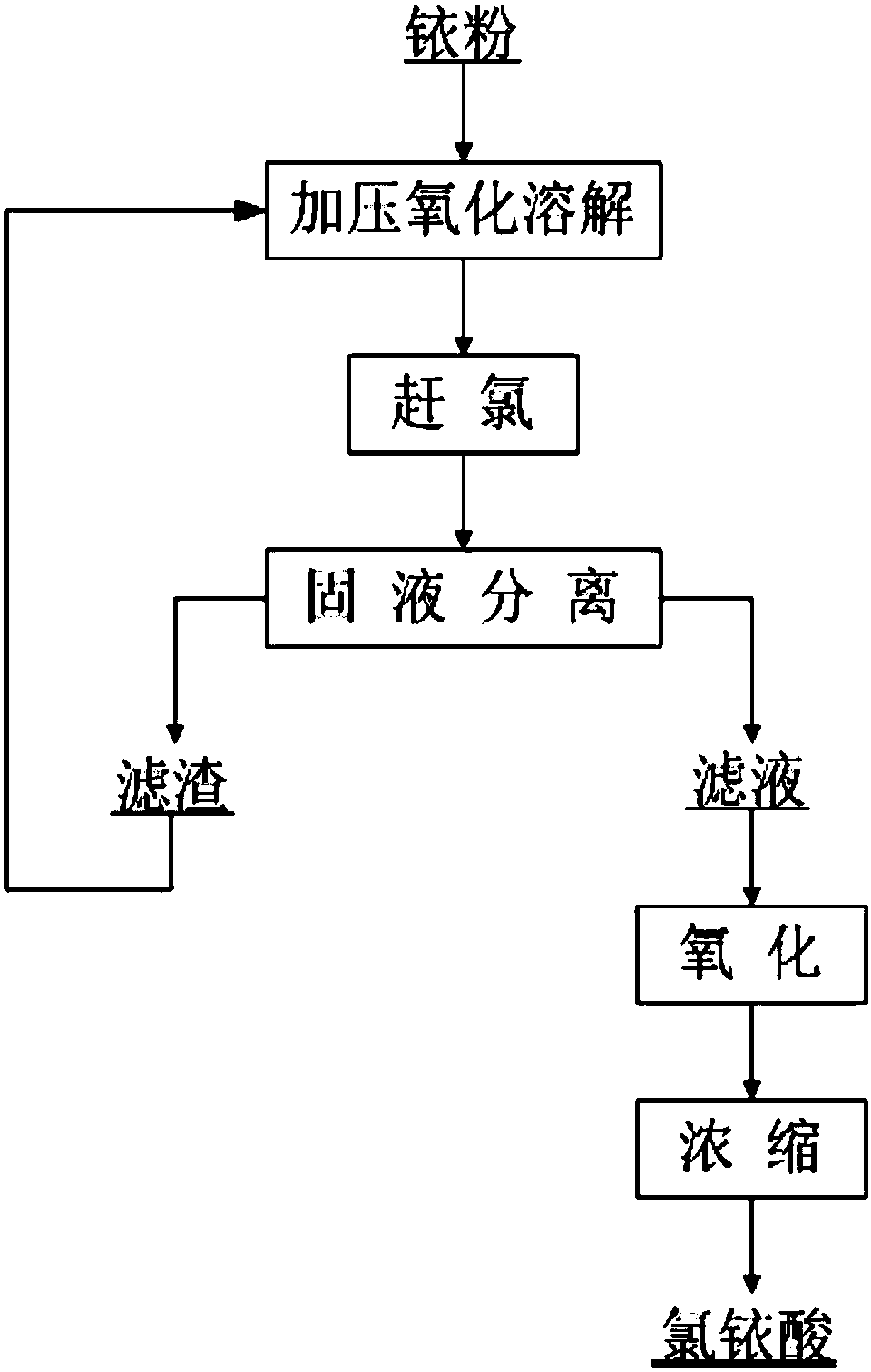
[0029] like figure 1 Shown, the method for preparing chloroiridic acid rapidly in the present embodiment may further comprise the steps:
[0030] Step 1, pressurized oxidation and dissolution: add 2kg of iridium powder and hydrochloric acid with a mass concentration of 36% into the reactor and feed chlorine gas while stirring. Stirring reaction 3h under the condition of min, stop feeding chlorine after reaction finishes, the flow of described chlorine is 800mL / min; The mass content of iridium in the described iridium powder is greater than 99.95%, the volume of described hydrochloric acid and the quality of iridium powder The ratio is 8mL: 1g;
[0031] Step 2, chlorine removal: after the reaction in step 1, open the pressure relief valve of the reactor, reduce the pressure of the reactor to normal pressure, and use the negative pressure system of the reactor to reduce the temperature in the reactor to 70°C , then add 5 times of concentrated hydrochloric acid with a total volum...
Embodiment 2
[0036] like figure 1 Shown, the method for the rapid preparation of chloroiridic acid of the present embodiment may further comprise the steps:
[0037] Step 1, pressurized oxidation and dissolution: add 2kg of iridium powder and hydrochloric acid with a mass concentration of 25% into the reactor and feed chlorine gas while stirring. The temperature in the reactor is 150°C, the pressure is 0.6MPa, and the rotation speed is 160r Stirring reaction 5h under the condition of / min, stop feeding chlorine gas after the end of reaction, the flow of described chlorine gas is 500mL / min; The mass content of iridium in the described iridium powder is greater than 99.95%, the volume of described hydrochloric acid and the quality of iridium powder The ratio is 4mL: 1g;
[0038] Step 2, chlorine removal: after the reaction in step 1, open the pressure relief valve of the reactor, reduce the pressure of the reactor to normal pressure, and use the negative pressure system of the reactor to re...
Embodiment 3
[0043] like figure 1 Shown, the method for the rapid preparation of chloroiridic acid of the present embodiment may further comprise the steps:
[0044] Step 1, pressurized oxidation and dissolution: add 2kg of iridium powder and hydrochloric acid with a mass concentration of 15% into the reactor and feed chlorine gas while stirring. The temperature in the reactor is 100°C, the pressure is 0.1MPa, and the rotation speed is 120r Under the condition of stirring reaction 8h under the condition of / min, stop feeding chlorine gas after the end of reaction, the flow rate of described chlorine gas is 200mL / min; The ratio is 2mL: 1g;
[0045] Step 2, chlorine removal: after the reaction in step 1, open the pressure relief valve of the reactor, reduce the pressure of the reactor to normal pressure, and use the negative pressure system of the reactor to reduce the temperature in the reactor to 85°C , then add concentrated hydrochloric acid with a total volume of 0.5L twice, each time ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com