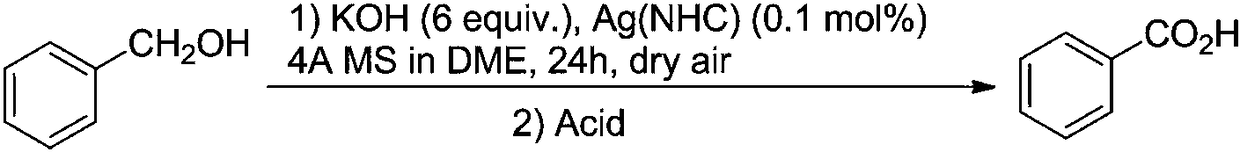
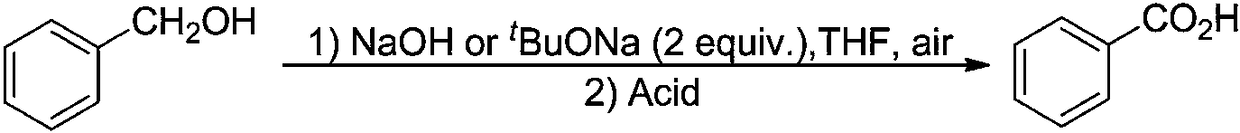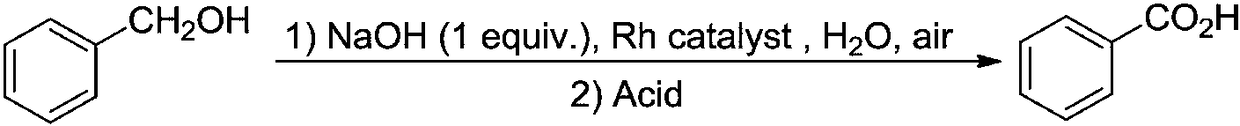Method for synthesizing benzoic acid compound from benzyl alcohol compound by ultrasonic-assisted oxidation
A technology for benzoic acids and compounds, which is applied in the field of organic intermediate synthesis, can solve the problems of incompatibility with alkali-sensitive functional groups, adverse environmental effects, and increase reaction costs, thereby reducing separation and purification costs, shortening reaction time, and low production costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Embodiment 1: the preparation of benzoic acid:
[0068] Into a 10mL round-bottomed flask, add 0.54g (5mmoL) of benzyl alcohol and 2g (15mmoL) of diethylene glycol dimethyl ether in sequence. The resulting mixture is subjected to ultrasonic radiation at 40KHz / 30W / 70°C for 30 minutes in an ultrasonic reaction device. . Diethylene glycol dimethyl ether was removed under reduced pressure, and 0.59 g of the corresponding benzoic acid was obtained by recrystallization, with a yield of 98%.
[0069] Scale-up preparation:
[0070] 1L three-neck round bottom flask, the middle grinding port is connected to a mechanical stirrer, one grinding port is externally connected to an 80cm serpentine condenser, and one external port is connected to an air source. Add 54g of benzyl alcohol and 200g of diethylene glycol dimethyl ether in sequence, and the resulting mixture is ultrasonically In the reaction device, 40KHz / 80W / 70°C ultrasonic radiation was used for 50 minutes. Diethylene gly...
Embodiment 2
[0075] Compared with Example 1, the difference is that the conversion reaction temperature is 60°C, specifically as follows:
[0076] Into a 10mL round bottom flask, add 0.54g of benzyl alcohol and 2g of diethylene glycol dimethyl ether in sequence, and react the resulting mixture in an ultrasonic reaction device with ultrasonic radiation at 40KHz / 30W / 60°C for 30 minutes. Diethylene glycol dimethyl ether was removed under reduced pressure, and 0.35 g of benzoic acid was obtained by recrystallization, with a yield of 58%.
Embodiment 3
[0078] Into a 10 mL round bottom flask, add 0.54 g of benzyl alcohol and 2 g of diethylene glycol dimethyl ether in sequence, and react the resulting mixture in an ultrasonic reaction device under ultrasonic radiation at 17KHz / 30W / 70°C for 30 minutes. Diethylene glycol dimethyl ether was removed under reduced pressure, and 0.54 g of benzoic acid was obtained by recrystallization, with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com