Synthesis method of m-xylylene diisocyanate (MXDI)
A technology of xylylene diisocyanate and synthetic method, which is applied to the preparation of isocyanic acid derivatives, purification/separation of isocyanic acid derivatives, chemical instruments and methods, etc., can solve the problems of increasing raw material costs, and achieve reduction The effect of production investment cost, equipment requirement reduction and strong market competitiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
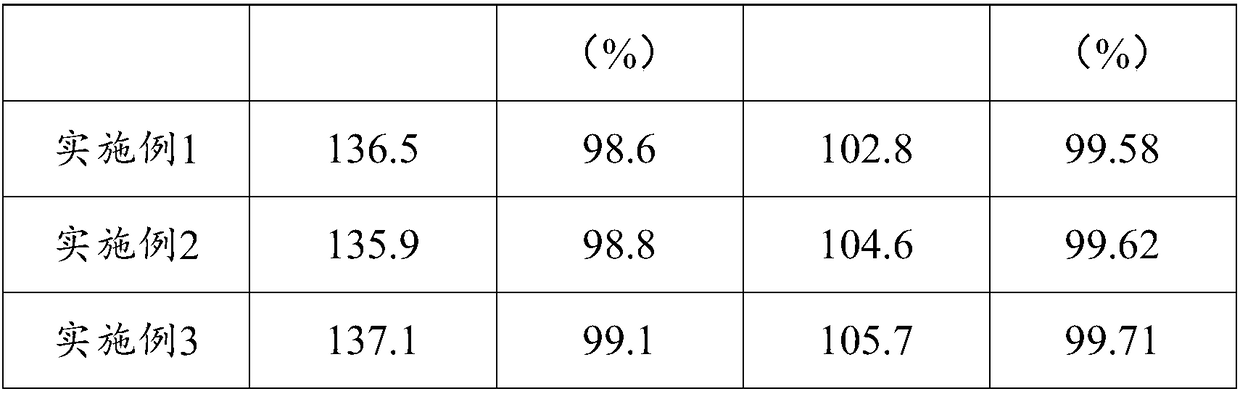
Embodiment 1
[0036] (1) 150g of solid phosgene was weighed and dissolved in 300g of chlorobenzene to obtain the first solid phosgene solution, which was heated to 55°C for subsequent use;
[0037] (2) Take by weighing 100g m-xylylenediamine (MXDA) and dissolve it in 200g chlorobenzene to obtain m-xylylenediamine solution;
[0038] (3) Add the m-xylylenediamine solution in step (2) dropwise to the first solid phosgene solution in step (1) within 2 hours at 55°C. During the dropwise addition, the precipitate under stirring state gradually generate;
[0039] (4) After the dropwise addition, the temperature was raised to 80° C. at a heating rate of 15° C. / h and kept for 2 hours, and then the temperature was raised to the reflux temperature within 2 hours, so that the reaction system was in a reflux state.
[0040] (5) 100g of solid phosgene was dissolved in 500g of chlorobenzene to obtain a second solid phosgene solution, and the second solid phosgene solution was added dropwise to the reacti...
Embodiment 2
[0045] (1) Weigh 150g of solid phosgene and dissolve it in an inert solvent composed of chlorobenzene, dichlorobenzene and toluene with a total weight of 450g to obtain the first solid phosgene solution, which is heated to 60°C for subsequent use;
[0046] (2) Take 100g m-xylylenediamine (MXDA) and dissolve it in the inert solvent of 400g to obtain m-xylylenediamine solution;
[0047] (3) Add the m-xylylenediamine solution in step (2) dropwise to the first solid phosgene solution in step (1) within 2.5 hours at 60°C, and precipitate under stirring during the dropwise addition things are gradually formed;
[0048] (4) After the dropwise addition, the temperature was raised to 90° C. at a heating rate of 17° C. / h and kept for 2.5 hours, and then the temperature was raised to reflux temperature within 2 hours, so that the reaction system was in a reflux state.
[0049] (5) Dissolving 100 g of solid phosgene in 700 g of the inert solvent to obtain a second solid phosgene solution...
Embodiment 3
[0054] (1) Weigh 150g of solid phosgene and dissolve it in an inert solvent composed of chlorobenzene, dichlorobenzene, toluene and xylene with a total weight of 750g to obtain the first solid phosgene solution, which is heated to 70°C for subsequent use;
[0055] (2) Take 100g m-xylylenediamine (MXDA) and dissolve it in the inert solvent of 500g to obtain m-xylylenediamine solution;
[0056] (3) Add the m-xylylenediamine solution in step (2) dropwise to the first solid phosgene solution in step (1) within 3 hours at 70°C. During the dropwise addition, the precipitate gradually generate;
[0057] (4) After the dropwise addition, the temperature was raised to 100° C. at a heating rate of 20° C. / h and kept for 3 hours, and then the temperature was raised to the reflux temperature within 2 hours, so that the reaction system was in a reflux state.
[0058] (5) Dissolve 100g of solid phosgene in 1000g of the inert solvent to obtain a second solid phosgene solution, and drop the seco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com