N-doped porous carbon coated Fe and Co bi-metal nanoparticle catalyst and preparation method thereof
A bimetallic nano-catalyst technology, applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problem of poor ORR performance and OER performance, and can not provide enough activity for bimetallic doped catalysts. Unsatisfactory and other problems, to achieve the effect of simple hole making process, improved stability, and avoiding direct contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
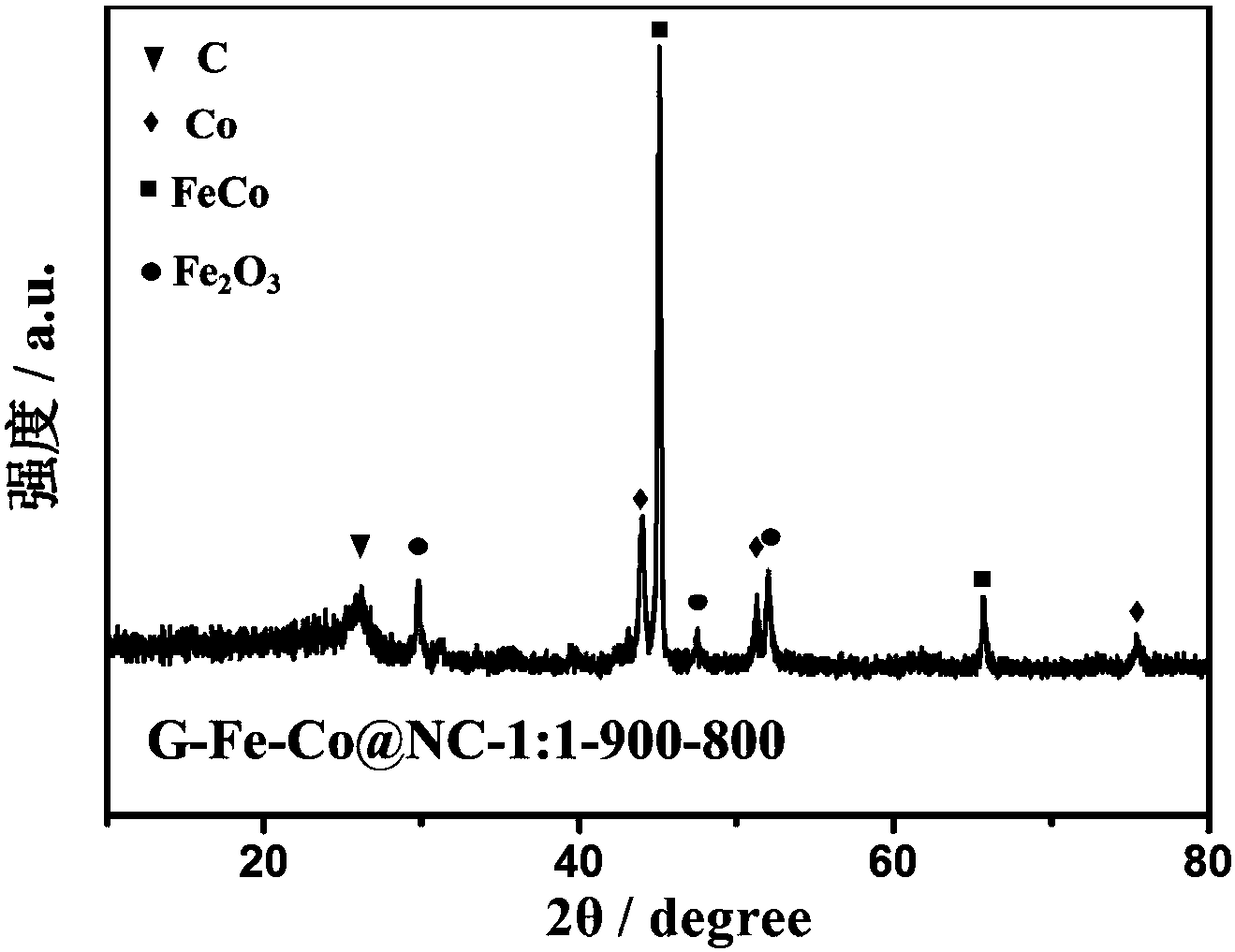
[0043] Example 1: G-Fe-Co@NC-1:1-900-800 (G is glucose, Fe-Co@NC-1:1 refers to FeCl in the raw material 3 ·6H 2 O and Co(NO 3 ) 2 ·6H 2 The molar mass ratio of O is 1:1 and FeCl is added first 3 ·6H 2 (O, 900 means the first pyrolysis temperature is 900°C, 800 means the second pyrolysis temperature is 800°C)
[0044] Put urea in a tube furnace under nitrogen protection at 5°C for min -1 Calcined for 4 hours at a temperature of 550°C to obtain g-C 3 N 4 ; Mix 20ml of water and 10ml of ethanol to obtain solution A, take 100mg of g-C 3 N 4 , 125mg FeCl 3 ·6H 2 O and 500 mg of glucose were dissolved in solution A, and then heated in an oil bath at 80°C for 8 hours to obtain solution B, and the uniformly mixed solution B was transferred to a petri dish, and dried in an air oven at 80°C for 10 hours to obtain a catalyst precursor① . Put the catalyst precursor ① in a mortar, grind it evenly, put it in a quartz boat, and put it under the protection of nitrogen at 5°C for ...
Embodiment 2
[0045] Example 2: G-Fe-Co@NC-2:1-900-800 (G is glucose, Fe-Co@NC-2:1 refers to FeCl in the raw material 3 ·6H 2 O and Co(NO 3 ) 2 ·6H 2 The molar mass ratio of O is 2:1 and FeCl is added first 3 ·6H 2 (O, 900 means the first pyrolysis temperature is 900°C, 800 means the second pyrolysis temperature is 800°C)
[0046] Put urea in a tube furnace under nitrogen protection at 5°C for min -1 Calcined for 4 hours at a temperature of 550°C to obtain g-C 3 N 4 ; Mix 20ml of water and 10ml of ethanol to obtain solution A, take 100mg of g-C 3 N 4 , 250mg FeCl 3 ·6H 2 O and 500 mg of glucose were dissolved in solution A, and then heated in an oil bath at 80°C for 8 hours to obtain solution B, and the uniformly mixed solution B was transferred to a petri dish, and dried in an air oven at 80°C for 10 hours to obtain a catalyst precursor① . Put the catalyst precursor ① in a mortar, grind it evenly, put it in a quartz boat, and put it under the protection of nitrogen at 5°C for ...
Embodiment 3
[0047] Example 3: G-Fe-Co@NC-2:3-900-800 (G is glucose, Fe-Co@NC-2:3 refers to FeCl in the raw material 3 ·6H 2 O and Co(NO 3 ) 2 ·6H 2 The molar mass ratio of O is 2:3 and FeCl is added first 3 ·6H 2 (O, 900 means the first pyrolysis temperature is 900°C, 800 means the second pyrolysis temperature is 800°C)
[0048] Put urea in a tube furnace under nitrogen protection at 5°C for min -1 Calcined for 4 hours at a temperature of 550°C to obtain g-C 3 N 4 ; Mix 20ml of water and 10ml of ethanol to obtain solution A, take 100mg of g-C 3 N 4 , 125mg FeCl 3 ·6H 2 O and 500 mg of glucose were dissolved in solution A, and then heated in an oil bath at 80°C for 8 hours to obtain solution B, and the uniformly mixed solution B was transferred to a petri dish, and dried in an air oven at 80°C for 10 hours to obtain a catalyst precursor① . Put the catalyst precursor ① in a mortar, grind it evenly, put it in a quartz boat, and put it under the protection of nitrogen at 5°C for ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com