Composition used for improving immune function and application thereof
A technology of immune function and composition, applied in the field of medicine, can solve problems such as safety and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
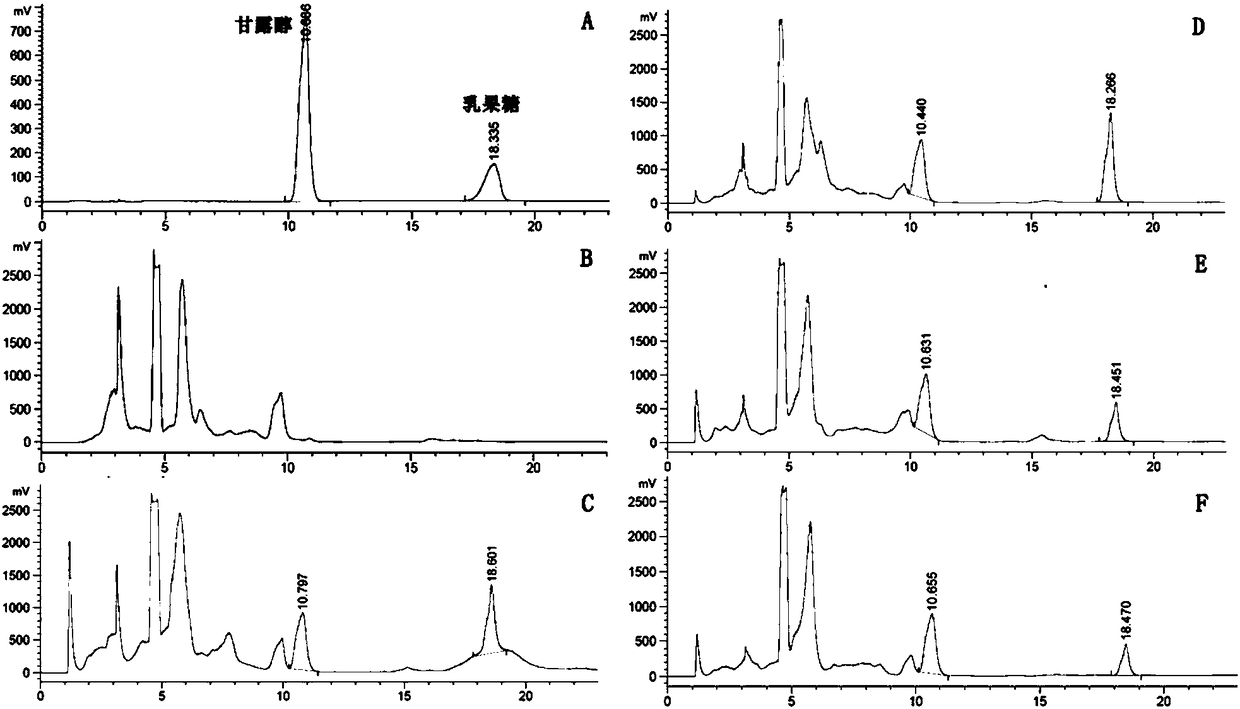
[0039] Embodiment 1: sample preparation
[0040] (1) Bacteria powder sample preparation: Activate the -80°C frozen glycerol tube strains of Lactobacillus plantarum HPL03, Lactobacillus rhamnosus HCL01, and Lactobacillus reuteri HCL04 on Rogosa solid medium, and culture them anaerobically at 37°C for 40 hours Pick a single colony and transfer it to a test tube containing 5ml of liquid medium (MRS Broth), and culture it anaerobically at 37°C for 24h; In the Erlenmeyer shake flask, anaerobic static culture at 37°C for about 18h; collect the fermentation broth, centrifuge at 4000rpm at 4°C for 10min, discard the supernatant, and wash twice with PBS (Cat. Finally, 20ml of PBS buffer was used to suspend the bacteria, and the concentrations of the three bacteria were controlled at 10 9 ~10 12 CFU / ml, mix, add equal volume of lyoprotectant liquid solution (8% skimmed milk powder, 3% lactose, 1% maltodextrin, 0.5% sodium glutamate, 0.5% ascorbic acid, the rest is water, and each subs...
Embodiment 2
[0043] Embodiment 2: animal experiments
[0044] (1) Animal grouping: BALB / c mice, SPF grade, provided by Beijing Weitong Lihua Experimental Animal Technology Co., Ltd., license number: SCXK (Beijing) 2012-0001; 40 BALB / c mice, 7 weeks Age, 20-22g, male, were randomly divided into 5 groups, 8 rats in each group, respectively control group, model group, test group 1, test group 2 and test group 3.
[0045] (2) Administration: The mice were administered by intragastric administration. The specific control group and model group administered PBS solution to the mice, and the test group administered the following samples to the mice: Test group 1: 4mg SA / Mice / day, test group 2: 4mg HA / mouse / day, test group 3: 4mg SHA / mouse / day. Specifically, 40 mg of samples were taken out from 4°C every day, adjusted to 5 ml with PBS buffer, mixed thoroughly, and 0.5 ml was administered to each mouse once a day for 21 consecutive days.
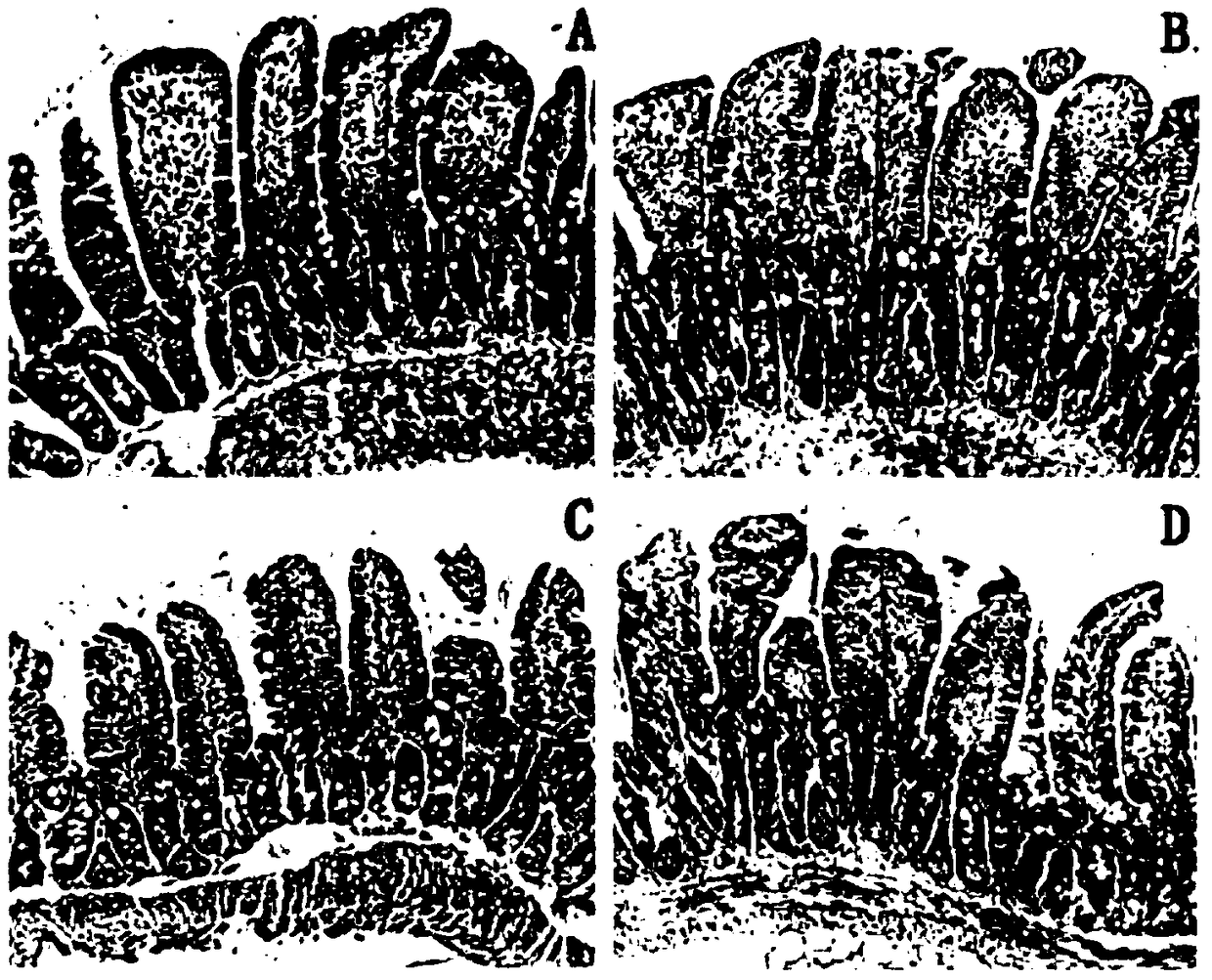
[0046] (3) Modeling: the animal model used in this test i...
Embodiment 3
[0047] Example 3: Detection of conventional immune indicators
[0048] (1) Detection method:
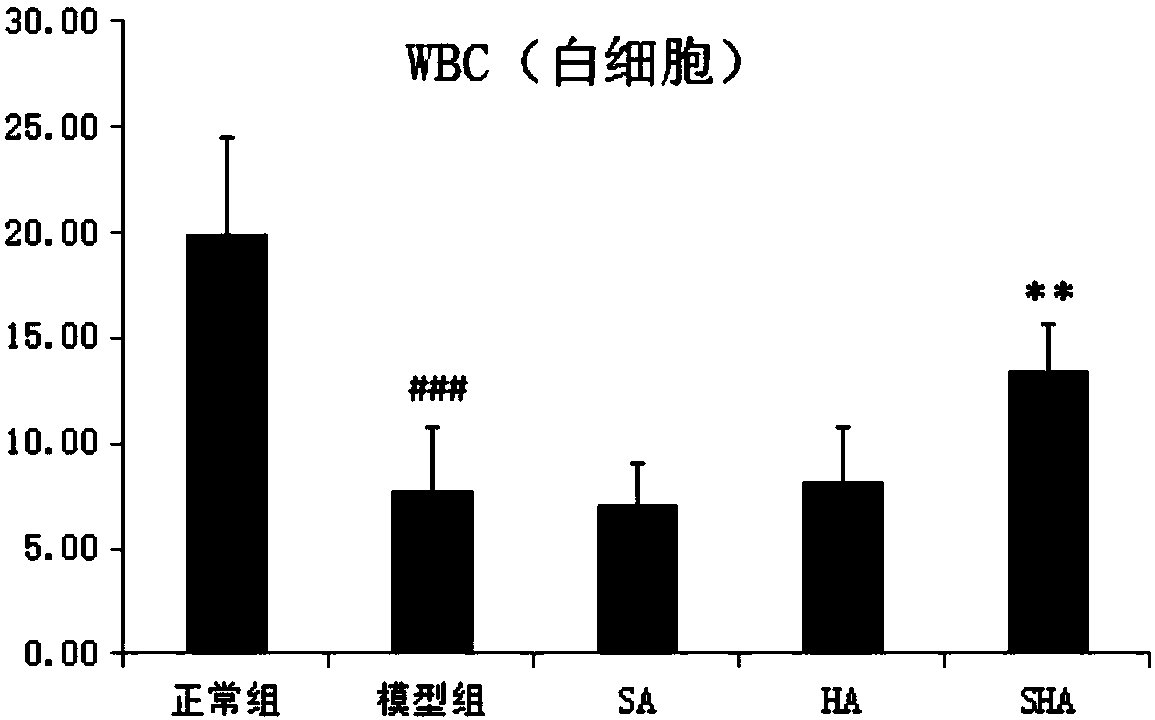
[0049] On the 5th day after modeling, use a blood cell analyzer to detect the number of red blood cells, platelets, white blood cells and lymphocytes in the tail vein blood of the mice; and take thymus and spleen, weigh them, and calculate the immune organ index.
[0050] Immune organ index = immune organ weight / body weight
[0051] (2) Statistical methods
[0052] The data obtained from the experiment are expressed as (MEAN±SD), and the differences among groups were compared by t test.
[0053] (3) Analysis of results:
[0054] A. The results of mouse organ analysis of each experimental group are shown in Table 1.
[0055] Table 1: Effects of different administrations on the immune organs of immunocompromised mice
[0056]
[0057] Note: a: The average molecular weight of HA is about 180,000-220,000 Daltons; b: the hyaluronic acid in the SHA sample is HA, and the average mol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com