Human IL2 (interleukin 2) and anti-EpCAM (epithelial cell adhesion molecule) single-chain antibody fusion protein and application thereof
A technology of fusion protein and epithelial cells, which is applied in the fields of genetic engineering, medicine and biomedical medicine, can solve the problems of drug entry, restriction of macromolecular antibody drugs and tumor immune cell tumor killing effect, difficulty of entry of immune cells and macromolecular drugs, etc. , to achieve the effects of reducing toxicity, promoting recognition and signal transduction, and better molecular penetration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Construction of fusion protein of anti-EpCAM single chain antibody and IL-2
[0050] The fusion protein EPIL2 of anti-EpCAM single-chain antibody and IL-2 comprises from N-terminal to C-terminal sequence: 1) IL-2 or IL-2 mutant; 2) linker; 3) anti-EpCAM single-chain antibody. The amino acid sequence of IL-2 or IL-2 mutant is shown in SEQ ID NO.2, 4, 6 and 8; The amino acid sequence of linker is shown in SEQ ID NO.10; The amino acid sequence of anti-EpCAM single chain antibody is shown in Shown in SEQID NO.11, 13.
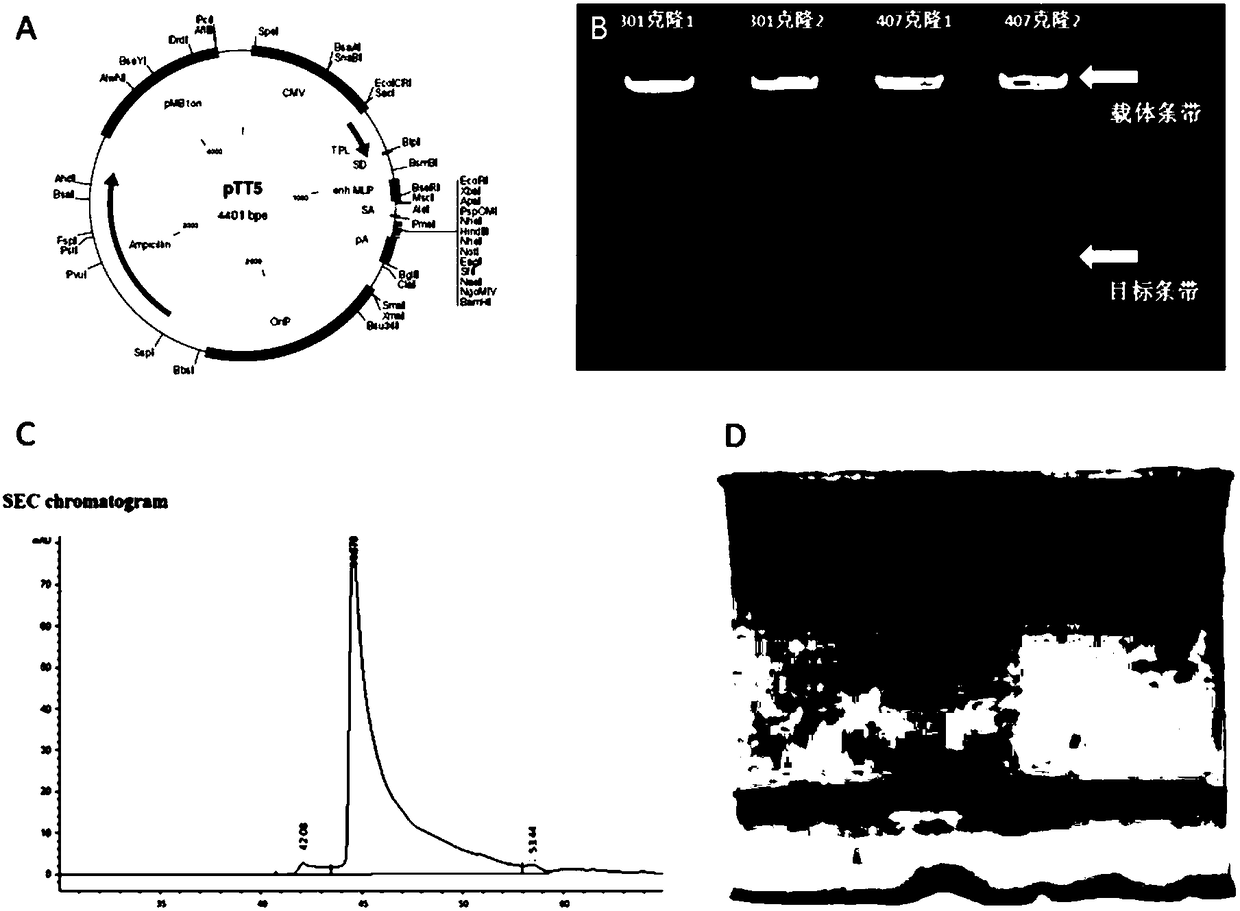
[0051] The DNA sequence of the fusion protein of the anti-EpCAM single-chain antibody and IL-2 is synthesized by the method of full-sequence gene synthesis (SEQ ID NO.16 and 18). After the synthetic sequence is inserted into the T vector, it is digested with HindIII and NheI pTT5 plasmid vector and pEASY-T1 positive clone loaded with target sequence. Recover the long fragment of the vector and the target fragment excised from the T vector, connec...
Embodiment 2
[0058] Example 2: Expression and purification of fusion protein of anti-EpCAM single chain antibody and IL-2
[0059] The low-endotoxin large-scale plasmid transfected HEK293 cells by liposome method, the specific steps are: inoculate 6x10 cells in 20ml DMEM (+10% FBS+antibiotics) medium 6 HEK293 cells. Cells were cultured at 37°C, 175rpm, 5% CO2 for 3 days. Take the cell count, when the cell density is about 2-3x10 6When cells / ml, dilute the treated cell density to 1x106 cells / ml with fresh DMEM (+10% FBS, no antibiotics) medium, and the volume of each bottle of cell liquid is 20mL, then tighten the bottle mouth and put it in a shaker to continue culturing. Transfection can be performed after 2-4 hours. In a sterile test tube, 500 microliters of DMEM was used to dilute 20 micrograms of plasmid, and in another test tube, 50ul of lipofectamine 2000 (brand: invitrogen product number: #11668-027) was added, and after standing for 5 minutes at room temperature, the plasmid was ...
Embodiment 3
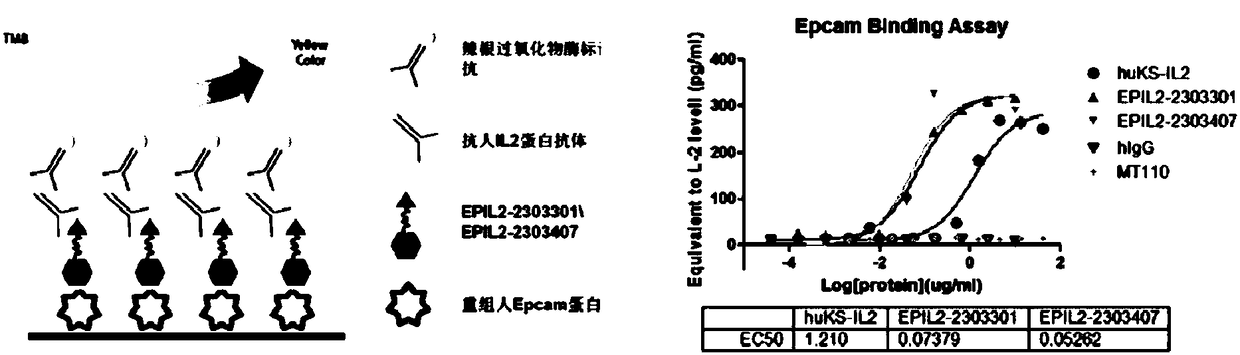
[0062] Example 3: Detection of the affinity activity of the fusion protein of anti-EpCAM single chain antibody and IL-2
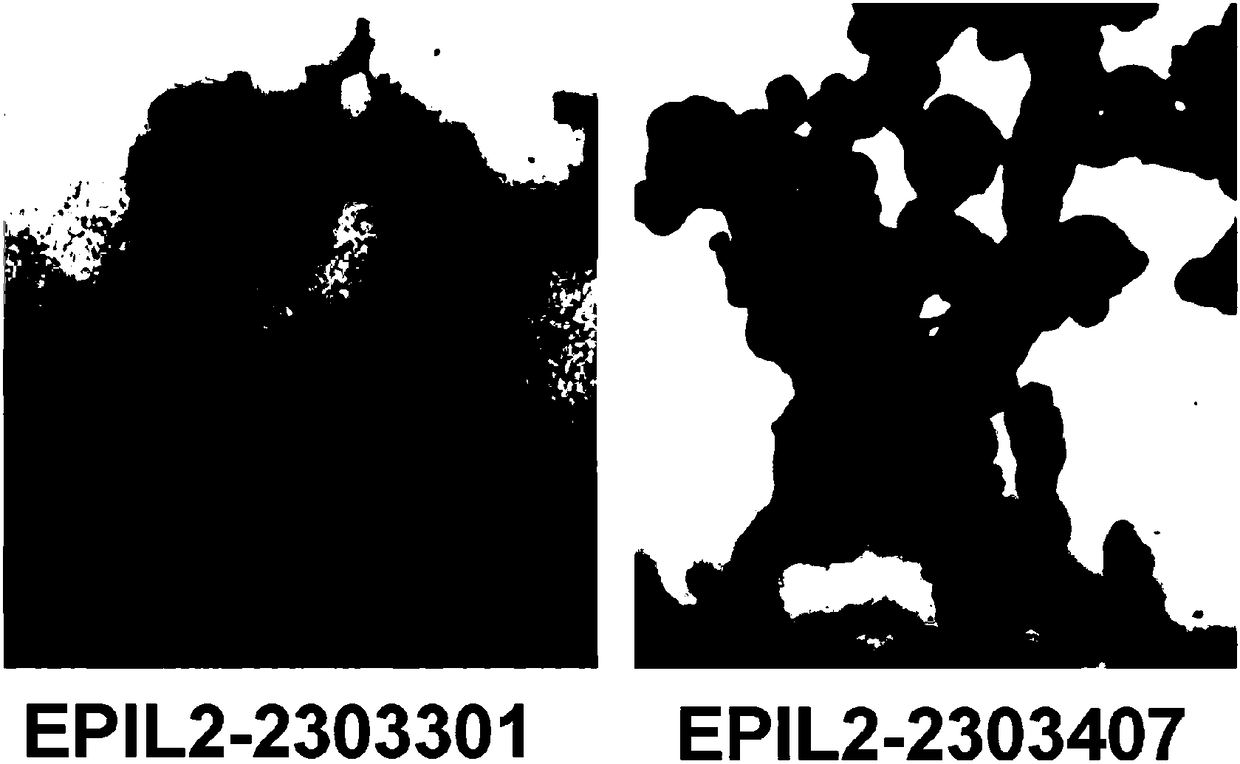
[0063] 1. Qualitative verification of the ability of the fusion proteins EPIL-2-2303301 and EPIL-2-2303407 to bind to cellular EpCAM:
[0064] skov3 cells were used for staining to test the affinity of EPIL-2-2303301 and EPIL-2-2303407 to EpCAM protein. The skov3 cells were grown adherently in a 24-well plate for 24 hours, fixed with 4% PFA (paraformaldehyde) for 10 minutes, added 5% BSA to block non-specific antigens, added anti-human IL-2 primary antibody and incubated for 1 hour, After washing with PBST for 3 times, the secondary antibody was added, and after incubation for 1 hour, DAB (diaminobenzidine) was added to develop the color. After washing with PBS, photographs were taken under a bright-field microscope. It can be seen that EPIL-2-2303301 and EPIL-2-2303407 can form strong cell staining, which qualitatively proves their ability to bind EpCAM a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com