Recombinant OmpK multi-epitope polypeptide, construction method and application thereof
A multi-epitope, 8-SEQIDNO technology, applied in the field of biomedicine, can solve the problems of poor immune effect and large side effects, and achieve the effect of improving resistance, high immunogenicity and reducing mortality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Embodiment 1 prepares nucleotide sequence
[0073] 1 Design and synthesis of OmpK protein epitope tandem gene
[0074] (1) Selection of OmpK protein amino acid sequence
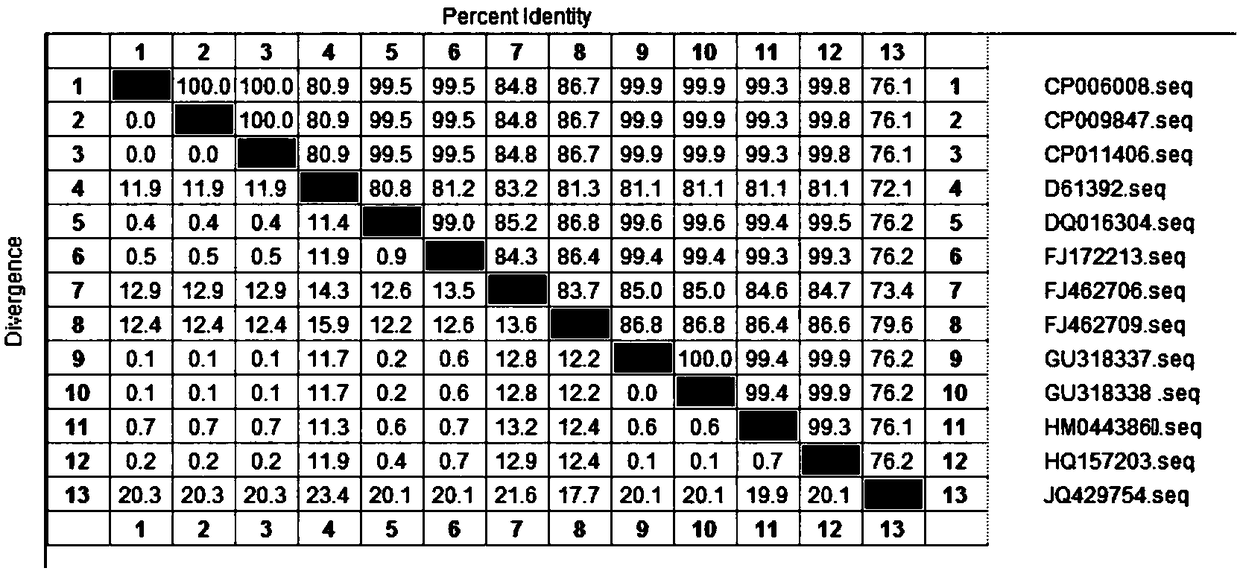
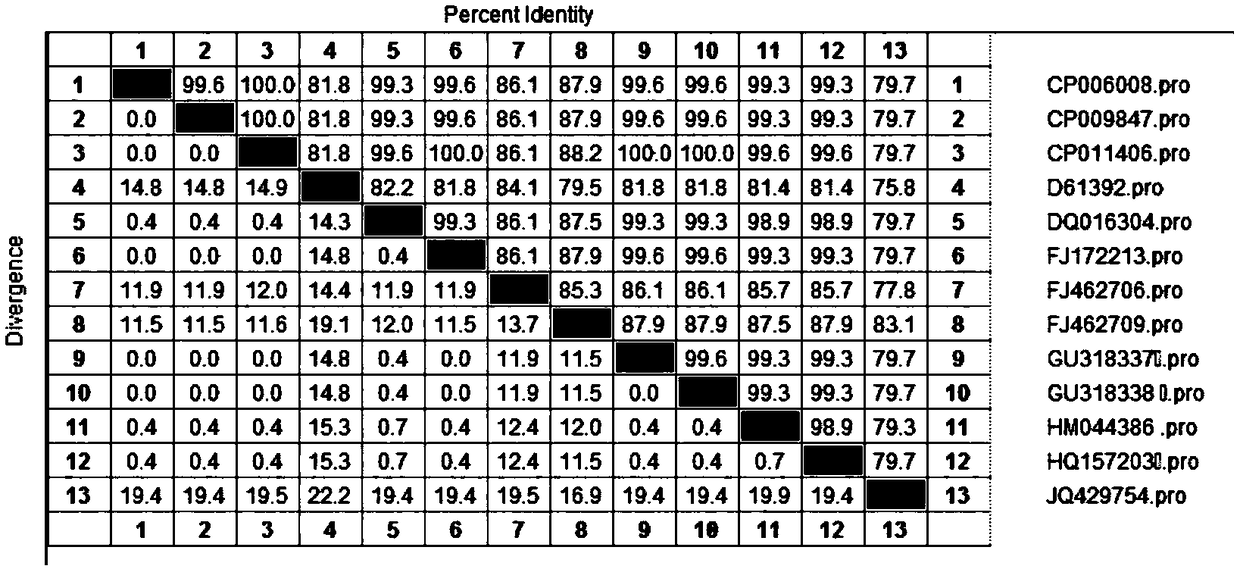
[0075] Log in the nucleotide sequence and amino acid sequence of the Vibrio parahaemolyticus OmpK gene searched in the National Center for Biological Information (NCBI) of the United States. After searching, there are 13 nucleotide sequences of the Vibrio parahaemolyticus OmpK registered by NCBI, such as As shown in Table 1, the length of the open reading frame (ORF) ranges from 792 to 822 bp, encoding 264 to 274 amino acids, and the molecular weight of OmpK protein is between 29.4KD to 30.5KD. The gene sequence accession number of the OmpK protein of ATCC17802 is HM044386.1 , ORF length is 819bp, encoding 273 amino acids, OmpK protein molecular weight is 30.3KDa. Using DNAStar Megalign software, the nucleotide and amino acid sequences of 13 OmpK genes were homologously compared and analyzed, and the...
Embodiment 2
[0100] Example 2 Preparation of expression plasmid
[0101] 1. Enzyme digestion reaction:
[0102] The nucleotide sequence (SEQ ID NO: 16) of the expressed rEPIS protein prepared in Example 1, the PCR product of the OmpK gene, and pET-32a(+) (product number: 69015-3; manufacturer: Novagen) were subjected to double enzyme digestion respectively , the enzyme digestion reaction system is: 4.5 μL of 10×K buffer, 1.5 μL each of BamHI and HindIII (Cat. No.: 1010S and 1060S, manufacturer: Baoriji Biotechnology (Beijing) Co., Ltd.), expressing the nucleotide sequence of rEPIS protein or OmpK gene PCR product or pET-32a(+) plasmid 20.0 μL (final concentration of both PCR products: 25ng / μL; plasmid final concentration: 32ng / μL), ddH2O 2.5 μL, total system 30.0 μl. React overnight in a water bath at 37°C, and use 0.8% agarose gel electrophoresis to detect the digested products. DNA gel recovery kit (article number: DP208; manufacturer: Tiangen Biochemical Technology (Beijing) Co., Ltd....
Embodiment 3
[0107] The preparation of embodiment 3 polypeptide / protein
[0108] 1 Preparation of recombinant OmpK epitope polypeptide (repis)
[0109] (1) Transformation of recombinant expression plasmids
[0110] Prepare E.coli Rosetta (DE3) competent cells according to the method in "Molecular Cloning Experiment Guide", take 2 tubes of 100 μL E.coli Rosetta (DE3) competent cells, and one of the tubes of competent cells is used as a control to coat LB agar plates Detect cell activity; add 20ng of the recombinant plasmid pET-32a-repis prepared in Example 2 to another tube of competent cells, mix well and then ice-bath for 30 minutes; after heat-shocking in a water bath at 42°C for 1 minute, quickly place it in an ice-bath for 1 minute ; Under sterile conditions, add 800μL of LB culture medium without Amp and shake culture (37℃, 160r / min) for 60min; centrifuge at 5,000r / min for 3min, discard the supernatant, then add 200μL of LB culture medium to resuspend the pellet, and take 100μL from ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com