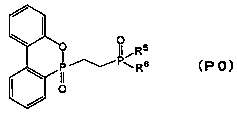
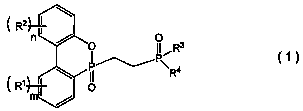
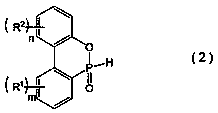
Organophosphorus compound and flame retardant agent comprising same, and method for producing organophosphorus compound
A manufacturing method and compound technology, applied in the field of organophosphorus compounds, can solve the problems of unfavorable implementation, low solubility, unfavorable economy, etc., and achieve the effect of high heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0145] Embodiment 1: Synthetic example (1) of organophosphorus compound
[0146] The following organophosphorus compound (P1) was synthesized by the following reaction.
[0147] [chemical formula 14]
[0148]
[0149] A flask with a capacity of 300 mL was prepared, and 50.0 g of DOPO was charged into the container to perform nitrogen substitution. 94.5 g of dimethyl vinylphosphonic acid (DMVP) was put into this container, and it reacted maintaining the temperature in the system at 130 degreeC (DOPO / DMVP=25 / 75 by molar ratio). After 8 hours from charging vinylphosphonic acid dimethyl, it was made to cool, and 96.3 g of toluene was added to the reaction liquid, and the product was deposited. The precipitated product was separated and recovered by filtration, and dried under reduced pressure to obtain 45.35 g of a white solid (99.38% yield). The resulting solid had a melting point of 138°C. In addition, formation of the compound (P1) was confirmed by 1H-NMR measurement of...
Embodiment 2
[0150] Embodiment 2: the synthesis example (2) of organophosphorus compound
[0151] The following organophosphorus compound (P2) was synthesized by the following reaction.
[0152] [chemical formula 15]
[0153]
[0154] A flask with a capacity of 500 mL was prepared, 120.0 g of DOPO was charged into the container, and nitrogen substitution was performed. 291.3 g of vinylphosphonic acid diphenyl ester (DPVP) was put into this container, and it reacted maintaining the temperature in the system at 130 degreeC (DOPO / DPVP=33 / 67 by molar ratio). After 8 hours from the injection of the vinylphosphonic acid diphenyl ester, it was allowed to cool, and the reaction liquid was injected into 1 L of acetone to deposit the product. The precipitated product was separated and recovered by filtration, and dried under reduced pressure to obtain 65.30 g of a white solid (yield 62.67%). The resulting solid had a melting point of 145°C. In addition, formation of the compound (P2) was co...
Embodiment 3
[0155] Embodiment 3: the synthesis example (3) of organophosphorus compound
[0156] Charge DOPO 0.25g, 9,10-dihydro-9-oxa-10-vinyl-10-phosphaphenanthrene-10-oxide (DOVP) 0.28g in the kugelrohr still (in terms of molar ratio DOPO / DOVP=50 / 50), decompressed to 10kPa, and heated at 200°C for 3 hours. Next, the temperature was raised to 230° C., followed by further heating for 1 hour, and unreacted DOVP was distilled off from the top of the still. Methanol was added to the bottom of the still, and the product was washed, separated by filtration, and dried under reduced pressure to obtain 0.48 g of a white solid (90% yield). The white solid was analyzed by 1H-NMR, and it was confirmed that it was the target 6,6'-(1,2-ethanediyl)bis[6H-dibenzo[c,e][1,2]oxaphosphapine Hexylcyclo-6-oxide] (EBDOPO).
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com