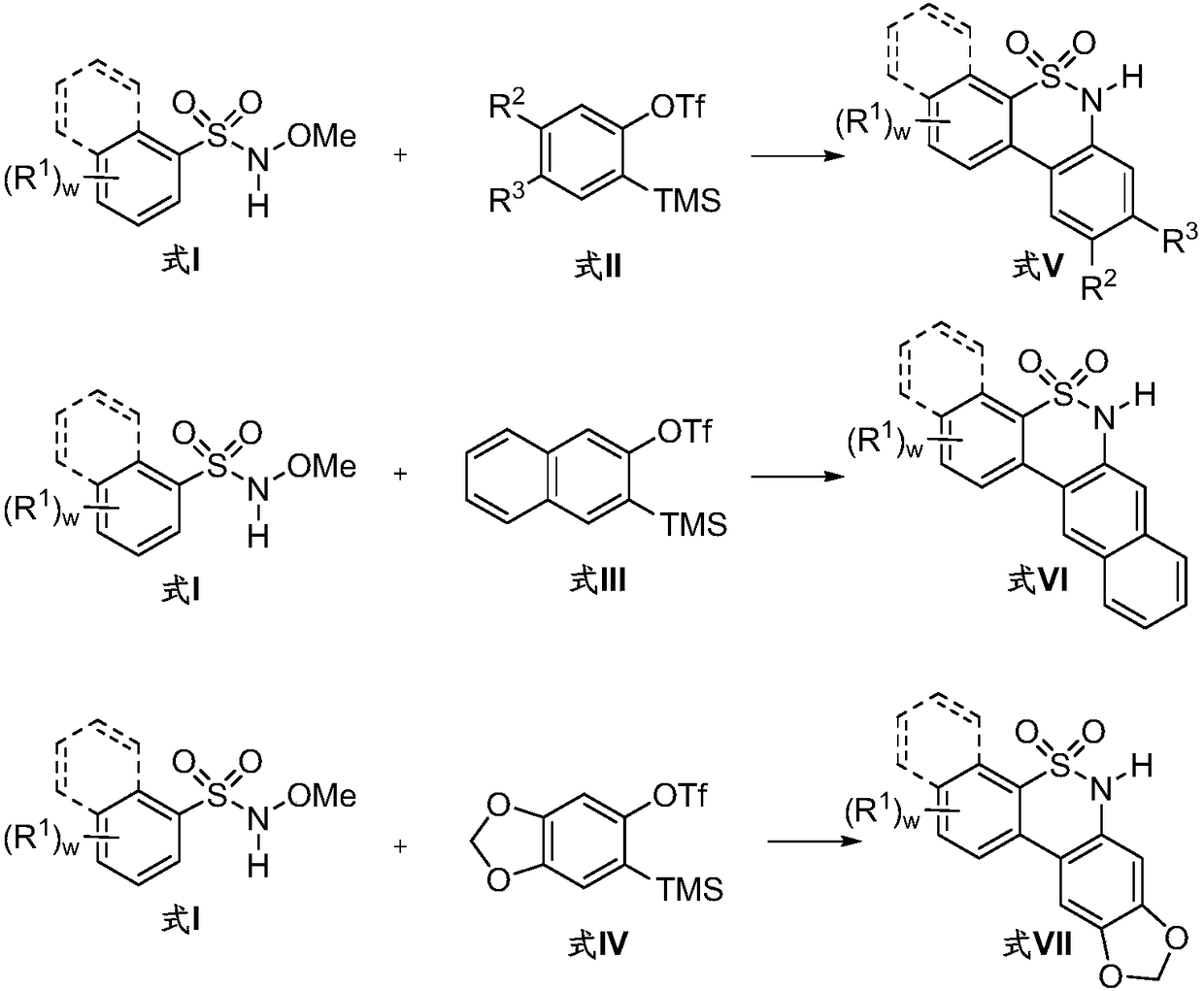
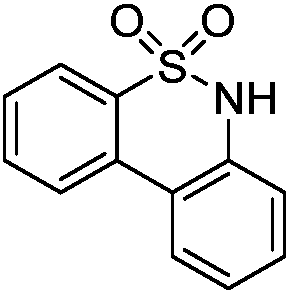
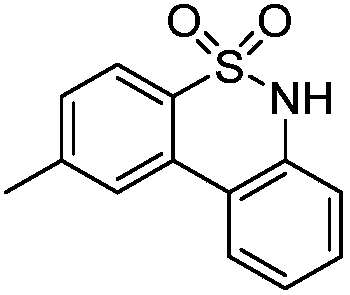
Synthetic method of diaryl sultam compounds
A technology of sultam and synthesis method, which is applied in the field of synthesis of diaromatic sultam compounds, can solve the problems of small applicable range of raw materials, harsh reaction conditions, etc., and achieves wide substrate applicability, simple reaction operation, and reaction Simple effect of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Taking the preparation of 6H-dibenzo[c,e][1,2]thiazine-5,5-dioxide with the following structural formula as an example, the raw materials used and the preparation method are as follows:
[0018]
[0019] Add 37.41mg (0.2mmol) N-methoxybenzenesulfonamide, 4.48mg (0.02mmol) palladium acetate, 72.7mg (0.4mmol) anhydrous copper acetate, 28.43mg (0.2mmol) in a 10mL pressure reaction tube in sequence Sodium pivalate hydrate, 100mg dry Molecular sieve, 121.52mg (0.8mmol) of cesium fluoride, 2.7mL of dioxane, 0.3mL of dimethyl sulfoxide, 119.3mg (0.4mmol) of benzene acetylene, under argon protection, a closed system at 110°C, stirring for 24h, reaction After the end, cool to room temperature, extract, combine the organic phases, anhydrous Na 2 SO 4 Dry and remove the organic solvent under reduced pressure. Dichloromethane is used as the eluent. The crude product is separated by silica gel column chromatography to obtain 6H-dibenzo[c,e][1,2]thiazine-5,5-dioxide The separation yiel...
Embodiment 2
[0022] Taking the preparation of 2-methyl-6H-dibenzo[c,e][1,2]thiazine-5,5-dioxide with the following structural formula as an example, the raw materials used and the preparation method are as follows:
[0023]
[0024] In Example 1, the N-methoxybenzenesulfonamide used was replaced with equimolar N-methoxy-4-methylbenzenesulfonamide, and the other steps were the same as in Example 1, to obtain 2-methyl-6H -Dibenzo[c,e][1,2]thiazine-5,5-dioxide, the separation yield is 83%, and the structural characterization data are as follows:
[0025] 1 H NMR(400MHz, DMSO-d 6 )δ (ppm) = 11.31 (s, 1H), 8.22 (d, J = 8.0 Hz, 1H), 8.09 (s, 1H), 7.84 (d, J = 8.0 Hz, 1H), 7.48 (t, J = 8.2Hz, 2H), 7.31 (t, J = 7.7 Hz, 1H), 7.22 (d, J = 8.0 Hz, 1H), 2.51 (s, 3H); 13 C NMR(100MHz, DMSO-d 6 )δ(ppm)=142.8,136.7,132.0,131.7,130.2,129.1,125.7,125.3,123.7,121.4,121.1,119.5,21.3; HRMS(ESI)m / z: C 13 H 11 NO 2 S[M+Na] + The theoretical value is 268.0403, and the measured value is 268.0393.
Embodiment 3
[0027] Taking the preparation of 2-tert-butyl-6H-dibenzo[c,e][1,2]thiazine-5,5-dioxide with the following structural formula as an example, the raw materials used and the preparation method are as follows:
[0028]
[0029] In Example 1, the N-methoxybenzenesulfonamide used was replaced with equimolar N-methoxy-4-tert-butylbenzenesulfonamide, and the other steps were the same as in Example 1, to obtain 2-tert-butyl -6H-dibenzo[c,e][1,2]thiazine-5,5-dioxide, the separation yield is 86%, and the structural characterization data are as follows:
[0030] 1 H NMR(400MHz, DMSO-d 6 )δ(ppm)=11.29(s,1H), 8.30(d,J=8.0Hz,1H), 8.14(s,1H), 7.86(d,J=8.2Hz,1H), 7.70(d,J= 8.3Hz, 1H), 7.46 (t, J = 7.7 Hz, 1H), 7.30 (t, J = 7.7 Hz, 1H), 7.20 (d, J = 8.0 Hz, 1H), 1.38 (s, 9H); 13 C NMR(100MHz,DMSO-d6)δ(ppm)=155.5,136.7,132.0,131.5,130.2,125.6,125.5,123.8,122.1,121.7,121.1,119.5,35.2,30.8; HRMS(ESI)m / z: C 16 H 17 NO 2 S[M+Na] + The theoretical value is 310.0872, and the measured value is 310.0858.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com