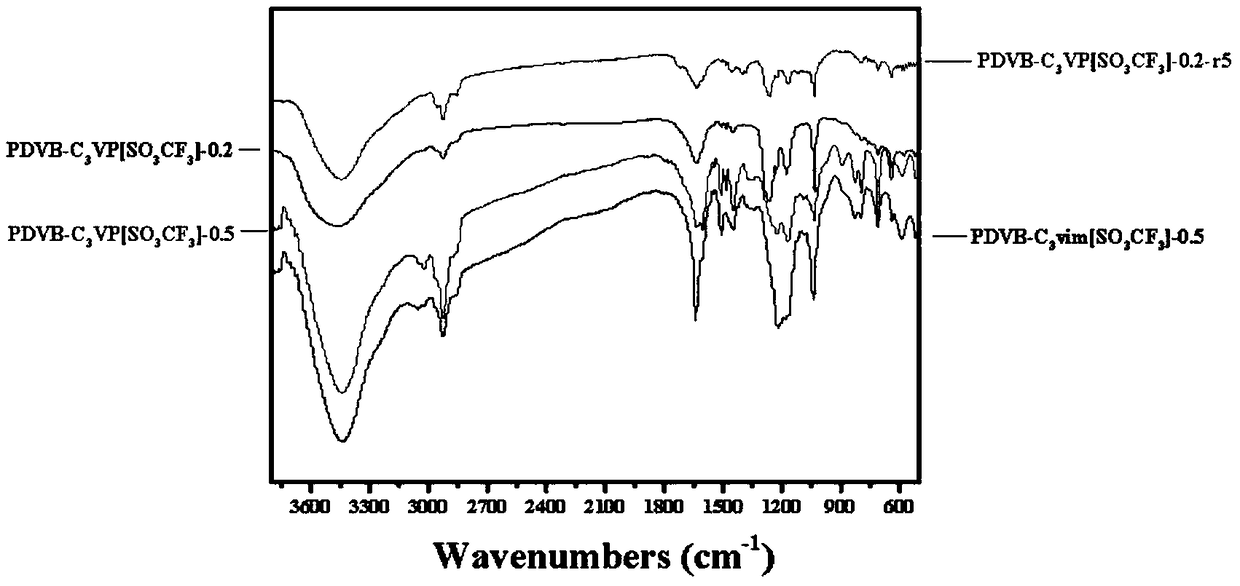
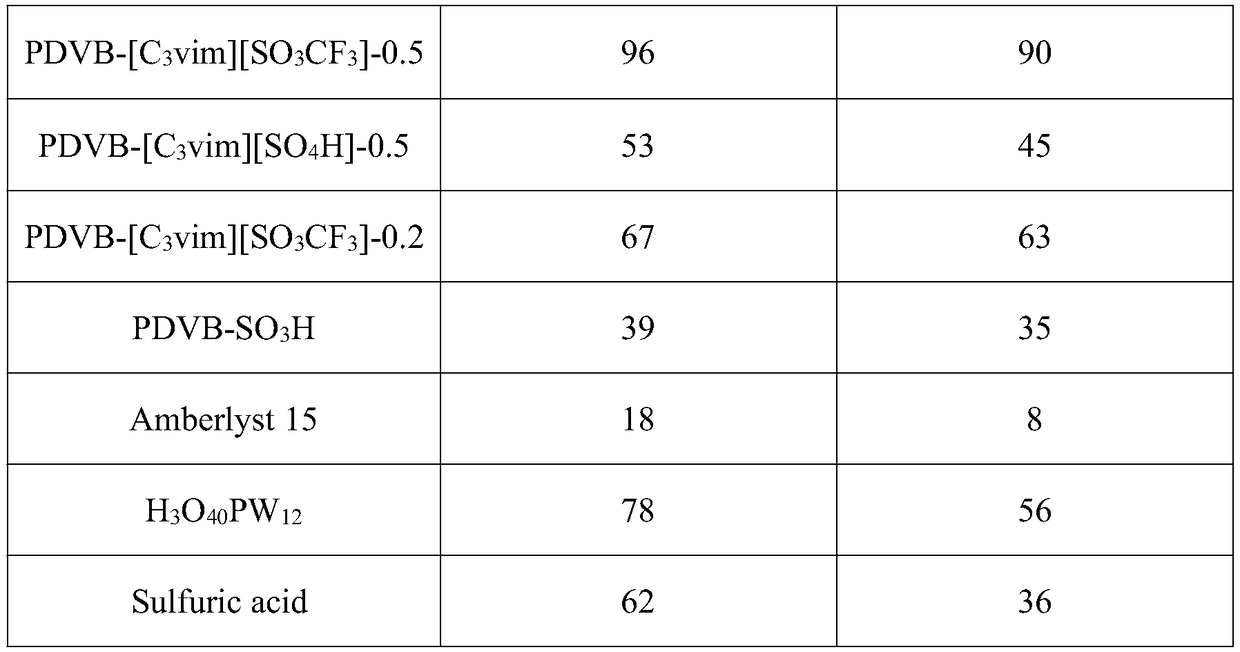
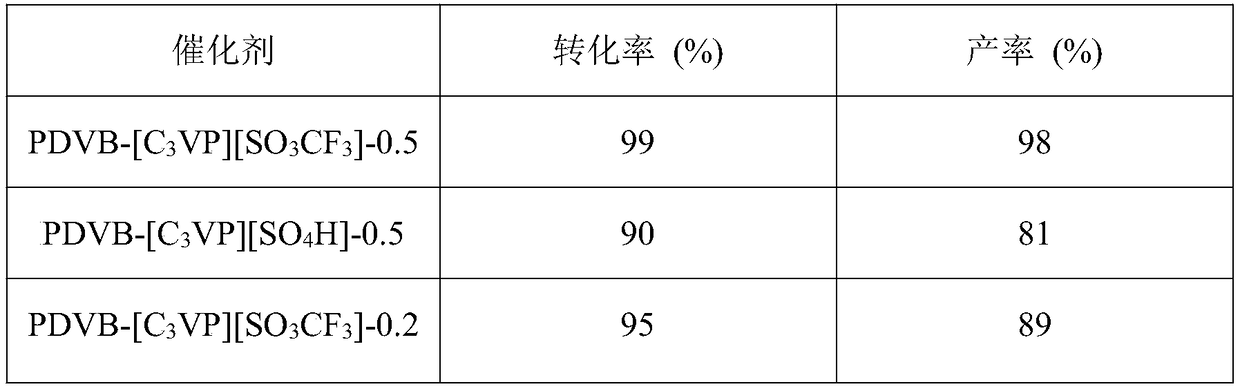
Porous polymer solid acid catalyst for alkyne hydration reaction
A solid acid catalyst and porous polymer technology, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, catalytic reactions, etc., can solve limited functional group compatibility, low acidic site concentration, Problems such as expensive and precious metals, to achieve the effects of controllable structure, easy operation, and enhanced acid strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A Porous Polymer Solid Acid Catalyst for Alkyne Hydration Reaction
[0027] Carrier synthesis: 2.0 g DVB and 0.5 g VP were added to a solution containing 0.07 g AIBN and 30 mL ethyl acetate. After stirring at room temperature for 12 hours, the mixture was solvent-treated at 120 °C for 24 hours, and then the solvent was slowly evaporated at room temperature for 2 days. A product with bulk morphology was obtained (PDVB-VP-0.5).
[0028] The prepared PDVB-VP-0.5 carrier was quaternized with 1,3-propane sultone, and then with HSO 3 CF 3 Perform ion exchange to synthesize PDVB-[C 3 VP][SO 3 CF 3 ]-0.5(C 3 represents a quaternizing agent for 1,3-propane sultone). Specifically, 1.0 g of PDVB-VP-0.5 was added to 25 mL of toluene under vigorous stirring, followed by the addition of 0.25 g of 1,3-propane sultone. After quaternization at 100°C for 24 hours, the product was collected by filtration, washed with copious amounts of ethanol and dried under vacuum at 80°C for 18...
Embodiment 2
[0030] A Porous Polymer Solid Acid Catalyst for Alkyne Hydration Reaction
[0031] Carrier synthesis: 2.0 g DVB and 0.2 g VP were added to a solution containing 0.07 g AIBN and 30 mL ethyl formate. After stirring at room temperature for 12 hours, the mixture was solvent-treated at 100 °C for 36 hours, and then the solvent was slowly evaporated at room temperature for 2 days. A product with bulk morphology was obtained (PDVB-VP-0.2).
[0032] The prepared PDVB-VP-0.2 carrier was quaternized with 1,3-propane sultone, and then with H 2 SO 4 Perform ion exchange to synthesize PDVB-[C 3 VP][SO 4 ]-0.2 (C3 represents the quaternization reagent of 1,3-propane sultone). Specifically, 1.0 g of PDVB-VP-0.2 was added to 35 mL of toluene under vigorous stirring, followed by the addition of 0.25 g of 1,3-propane sultone. After quaternization at 130 °C for 24 h, the product was collected by filtration, washed with copious amounts of ethanol and dried at 100 °C for 12 h. The resulting...
Embodiment 3
[0034] A Porous Polymer Solid Acid Catalyst for Alkyne Hydration Reaction
[0035]Carrier synthesis: 2.0 g DVB and 0.4 g vim were added to a solution containing 0.05 g AIBN and 30 mL methyl acetate. After stirring at room temperature for 24 hours, the mixture was solvent-treated at 100 °C for 36 hours, and then the solvent was slowly evaporated at room temperature for 2 days. A product with bulk morphology was obtained (PDVB-vim-0.4).
[0036] The prepared PDVB-vim-0.4 carrier was quaternized with 1,3-propane sultone, and then with HSO 3 CF 3 Perform ion exchange to synthesize PDVB-[C 3 vim][SO 3 CF 3 ]-0.4(C 3 represents a quaternizing agent for 1,3-propane sultone). Specifically, 1.0 g of PDVB-vim-0.4 was added into 35 mL of toluene under vigorous stirring, followed by the addition of 0.30 g of 1,3-propane sultone. After quaternization at 140°C for 36 hours, the product was collected by filtration, washed with copious amounts of ethanol and dried under vacuum at 80°C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com