Triphenylamine containing isoindigo polymer, preparation method thereof, and application to electrochromism
The technology of an electrochromic layer and an electrochromic device is applied to a triphenylamine group-containing isoindigo polymer and its preparation and application in electrochromism, which can solve the problem of low solubility of isoindigo and narrow application range of isoindigo And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
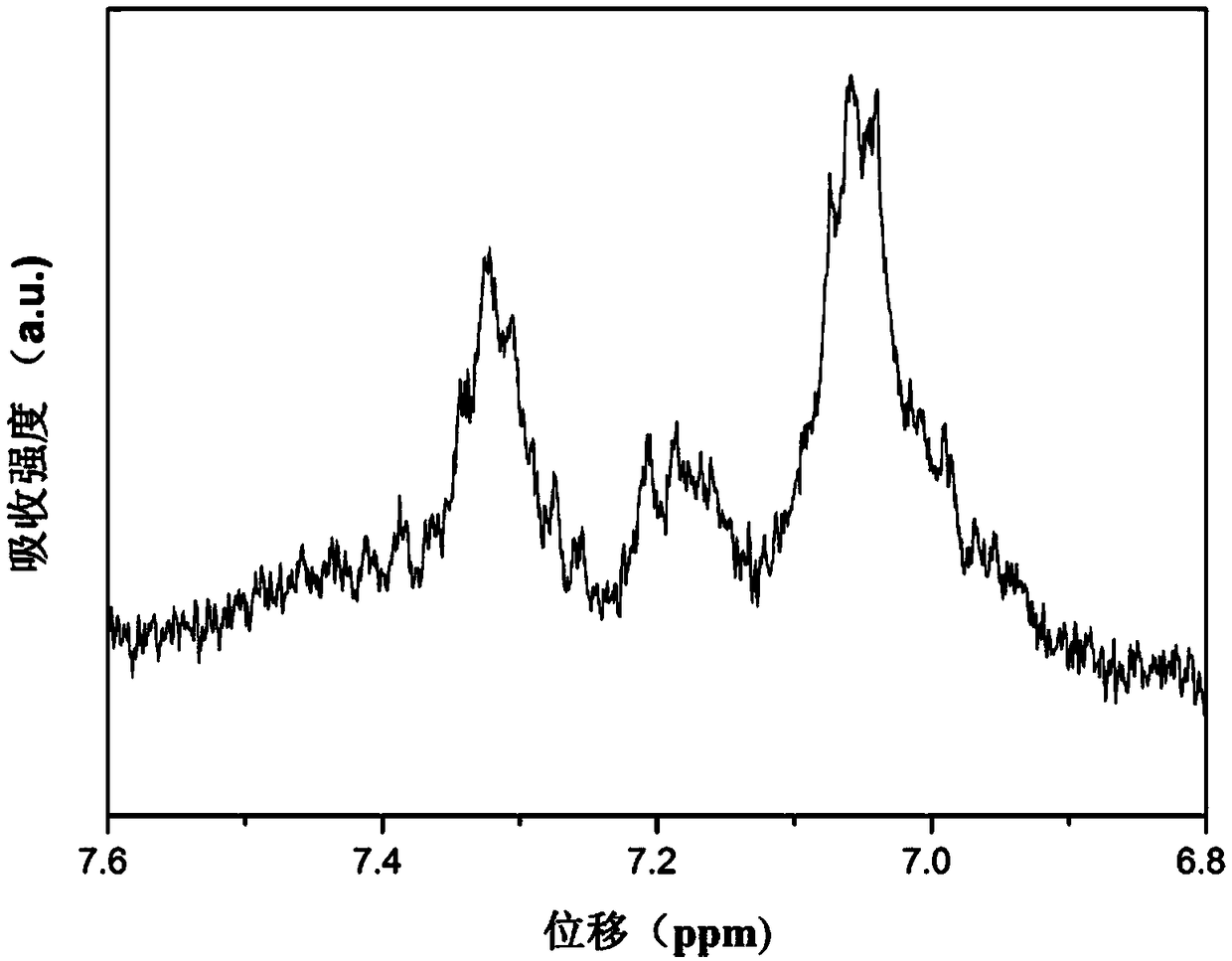
[0086] Specific embodiment one: the structural formula of this embodiment containing triphenylamino-isoindigo polymer is:
[0087] In the formula, n is a positive integer.
[0088] This embodiment has the following beneficial effects:
[0089] The isoindigo conjugated polymer containing triphenylamine group and isoindigo group in this embodiment increases the solubility of isoindigo due to the introduction of long alkyl chains, and is easily soluble in polar solvents. Soluble in 1-1.5 grams in medium; slightly soluble in non-polar solvents, soluble in 0.1-0.2 grams per 10 ml of polar solutions;
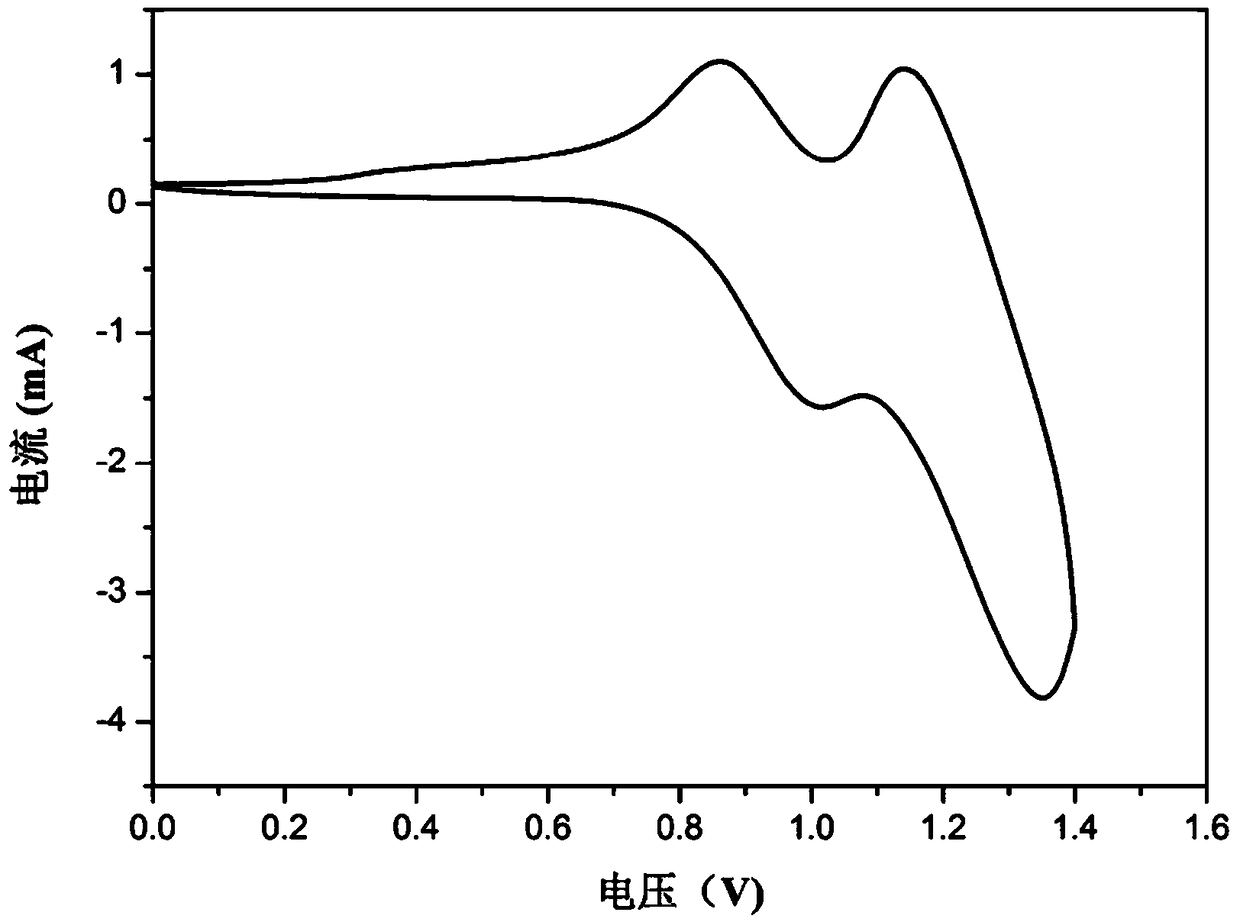
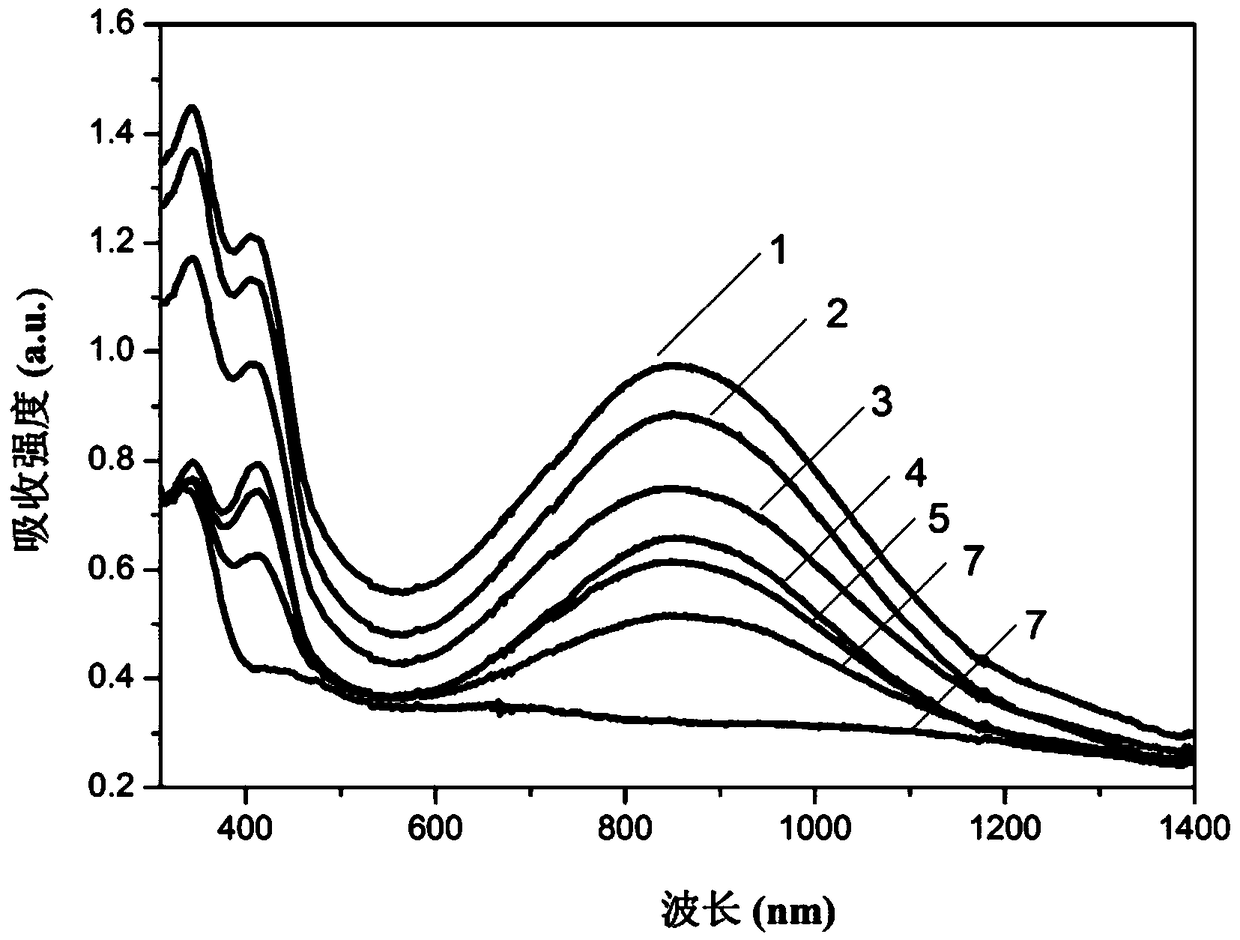
[0090] 2. The polymer in this embodiment has excellent electrochromic performance and memory performance, can be applied in the field of electrochromic, and it also has good performance in explosive detection and photoelectric detection;
[0091] Electrochromism refers to the phenomenon of color change caused by electrochemical redox reaction of substances under the drive of exter...
specific Embodiment approach 2
[0094] Specific embodiment two: present embodiment contains the preparation method of triphenylamino-based isoindigo polymer according to the following steps:
[0095] 1. Synthesis of N 1 , N 1 -Bis(4-bromophenyl)-N 4 , N 4 -Diphenylbenzene-1,4-diamine monomer:
[0096] ①, in N 2 Under the atmosphere, put diphenylamine monomer, sodium hydride and anhydrous N, N-dimethylformamide in a three-necked flask, stir while adding p-fluoronitrobenzene at a rate of 1-2 drops per second, and heat up to After 114~115℃, carry out constant temperature reaction, use thin layer chromatography to judge whether the constant temperature reaction is over, cool down after the constant temperature reaction is over; put the reaction product in cold water until the crude product is precipitated, then filter out the crude product, and wash the crude product with hot water 2 to 3 times, use ethanol to recrystallize the crude product washed with hot water, filter the crystalline product after recrys...
specific Embodiment approach 3
[0150] Specific embodiment three: this embodiment is different from specific embodiment two: the eluent used for the chromatographic column separation solid product described in step 2.2 is the mixed solution of dichloromethane and sherwood oil, dichloromethane and The volume ratio of petroleum ether is 1: (7-8). Other steps and parameters are the same as in the second embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com