Drug moisture-proof agent and preparation method and application thereof
A moisture-proof agent and drug technology, which is applied in the direction of pharmaceutical formulas, medical preparations of non-active ingredients, inorganic non-effective ingredients, etc., can solve problems such as limited application fields and insignificant moisture-proof effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of described pharmaceutical moistureproof agent, comprises the following steps:
[0030] S1: dissolving corn gluten in ethanol with a concentration of 95% to make a corn gluten solution with a concentration of 90%-95%, and simultaneously dissolving soluble starch, calcium hydrogen phosphate, and lactose with 50-60ml of hot water at 85-95°C, Then add the corn gluten solution and mix evenly to obtain the premix;
[0031] S2: Dissolve sucrose in 20-30ml of deionized water, add micro-powdered silica gel, stir well, and leave for 1-2 hours to obtain sucrose micro-powdered silica gel mixture;
[0032] S3: Mix the sucrose micropowder silica gel mixture, hydrophobic nano silicon dioxide, maltodextrin, magnesium stearate, hydroxypropyl cellulose, povidone, and then add microcrystalline cellulose and the premix to fully Mix evenly, and then put it into a high-speed mixer to fully stir evenly, adjust the speed of the mixer to 2000-2500r / min, and stir for 15...
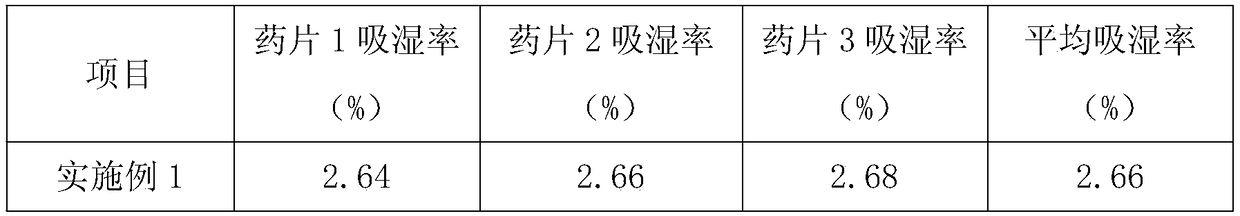
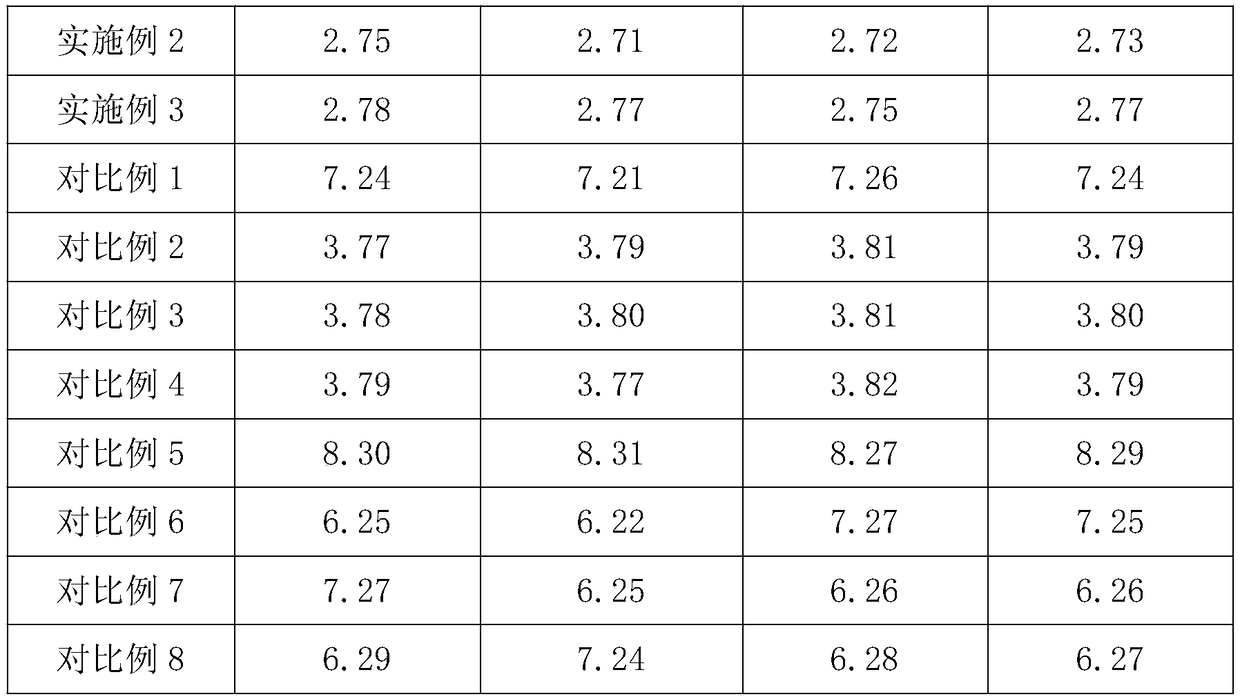
Embodiment 1
[0035]A pharmaceutical moisture-proof agent, in parts by weight, comprising the following raw materials: 28 parts of corn gluten, 5 parts of soluble starch, 1.5 parts of calcium hydrogen phosphate, 0.5 parts of lactose, 0.1 part of magnesium stearate, 1.3 parts of microcrystalline cellulose, 0.6 parts of hydroxypropyl cellulose, 0.5 parts of povidone, 0.2 parts of sucrose, 1.2 parts of micronized silica gel, 0.9 parts of hydrophobic nano silicon dioxide, and 0.9 parts of maltodextrin.
[0036] The preparation method of described pharmaceutical moistureproof agent, comprises the following steps:
[0037] S1: Dissolve corn gluten in ethanol with a concentration of 95% to make a corn gluten solution with a concentration of 90%. At the same time, stir soluble starch, calcium hydrogen phosphate, and lactose to dissolve in 50ml of 95°C hot water, then add corn gluten solution and mix well , to obtain the premix;
[0038] S2: Dissolve sucrose in 20ml of deionized water, add micropow...
Embodiment 2
[0042] A pharmaceutical moisture-proof agent, in parts by weight, comprising the following raw materials: 20 parts of corn protein, 15 parts of soluble starch, 1 part of calcium hydrogen phosphate, 0.9 parts of lactose, 0.6 parts of magnesium stearate, 0.8 parts of microcrystalline cellulose, 1.4 parts of hydroxypropyl cellulose, 0.4 parts of povidone, 0.15 parts of sucrose, 1.12 parts of micronized silica gel, 0.62 parts of hydrophobic nano silicon dioxide, and 0.86 parts of maltodextrin.
[0043] The preparation method of described pharmaceutical moistureproof agent, comprises the following steps:
[0044] S1: Dissolve corn gluten in ethanol with a concentration of 95% to make a corn gluten solution with a concentration of 95%. At the same time, stir soluble starch, calcium hydrogen phosphate, and lactose to dissolve in 60ml of 90°C hot water, then add corn gluten solution and mix well , to obtain the premix;
[0045] S2: Dissolve sucrose in 30ml of deionized water, add mic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com