Phenothiazine derivative fluorescence carbon dots as well as preparation method and application thereof
A technology of phenothiazine derivatives and fluorescent carbon dots, applied in photodynamic therapy, chemical instruments and methods, nano-carbon, etc., can solve the problems of carcinogenic risk and DNA infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] In a further specific embodiment, the preparation method of the present invention includes the following steps:
[0030] (1) preparing an aqueous solution of a phenothiazine derivative with a concentration of 0.5-5 mg / mL;
[0031] (2) Add passivating agent to the solution prepared in step (1), naturally cool to room temperature after hydrothermal reaction;
[0032] (3) The product obtained in step (2) is centrifuged, and the obtained supernatant is processed to obtain fluorescent carbon dots of phenothiazine derivatives.
[0033] Wherein, the passivating agent described in step (2) is 1-30 mg / mL PEG200-2000 or 0.1-5 mg / mL urea. In this description, the concentration of the passivating agent represents the concentration of the passivating agent in the reaction system.
[0034] On the other hand, step (2) refers to the hydrothermal reaction of phenothiazine derivatives in the presence of a passivating agent, and the conditions of this reaction can be defined according t...
Embodiment 1
[0038] Preparation of methylene blue carbon dots: Weigh 15 mg of methylene blue into a 25 mL beaker, add 15 mL of deionized water and stir to dissolve it, then add 150 μL PEG800 into it with a pipette gun, and stir evenly. The resulting solution was transferred into a 50ml reactor at 180°C for 12 hours. After the reaction, cool to room temperature in the air, centrifuge at 8000r / min for 20 minutes to obtain the methylene blue carbon dot solution, store it at around 4°C, and use it for the following performance test experiments 1-5.
[0039] (1) Performance Test Experiment 1: Morphology analysis of methylene blue carbon dots.
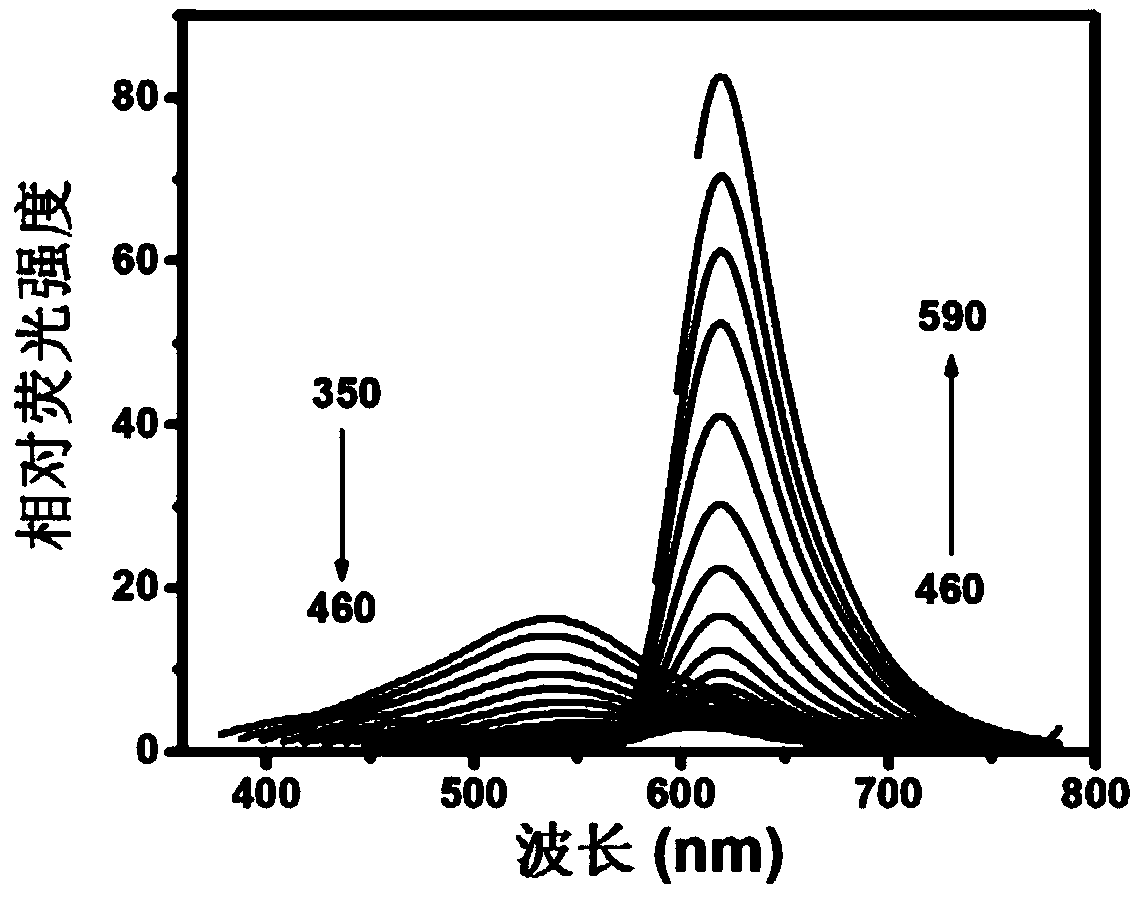
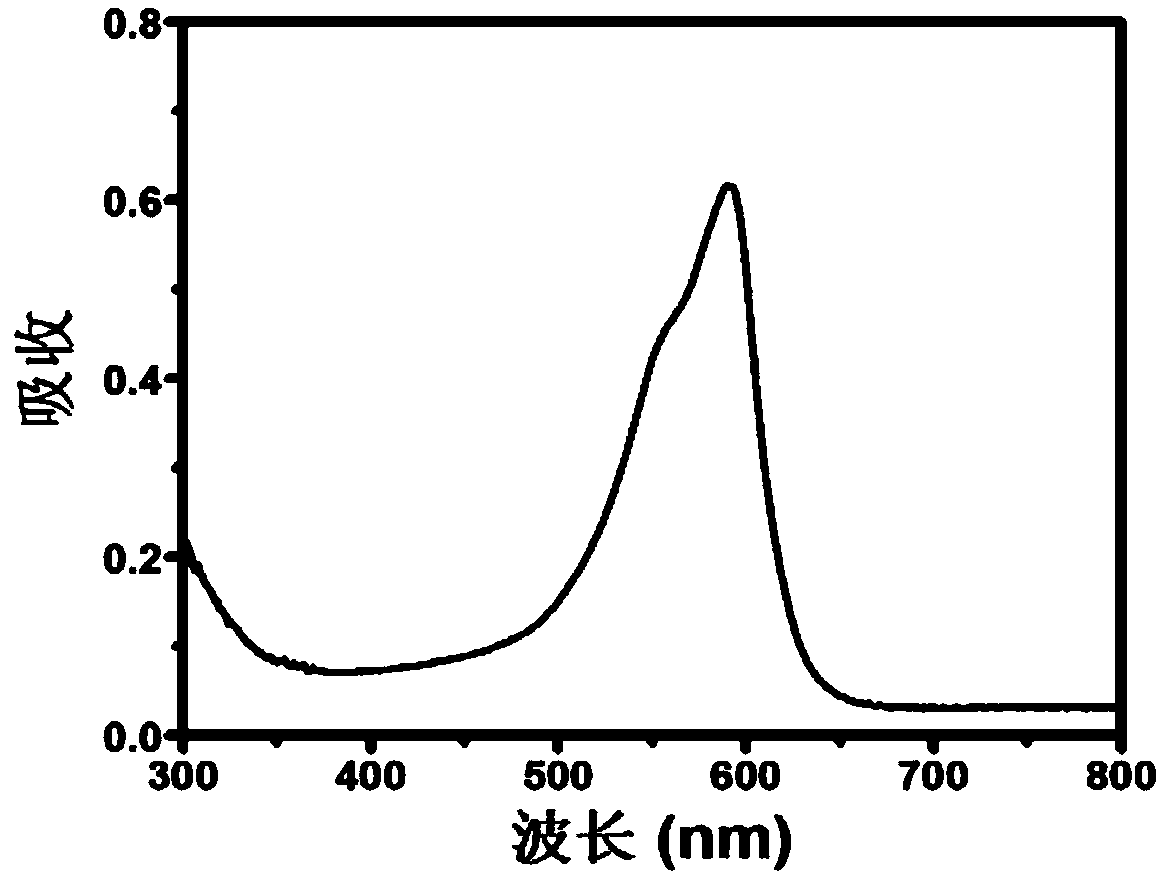
[0040] The methylene blue carbon quantum dot solution was taken, and the hydrated average particle size was measured by a Zeta potential and particle size analyzer to be 4.3nm, and the Zeta potential was -7.3V. Obtain methylene blue carbon dot TEM images (such as figure 1 ). figure 1 In the middle, the scale bar of the large picture is 20nm, and the i...
Embodiment 2
[0054] Preparation of toluidine blue (CAS registration number: 92-31-9) carbon dots: Weigh 15 mg of toluidine blue and place it in a 25 mL beaker, add 15 mL of deionized water and stir to dissolve it, then measure it with a pipette gun 200μL PEG800 was added and stirred evenly. The resulting solution was transferred into a 50ml reactor at 160°C for 8 hours of reaction. After the reaction, cool to room temperature in the air, and centrifuge at a speed of 8000r / min for 20 minutes to obtain a toluidine blue carbon dot solution, which is stored at about 4°C until use.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com