Method for preparing formic acid by bipolar membrane electrodialysis
A bipolar membrane electrodialysis, bipolar membrane technology, used in the preparation of carboxylate, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
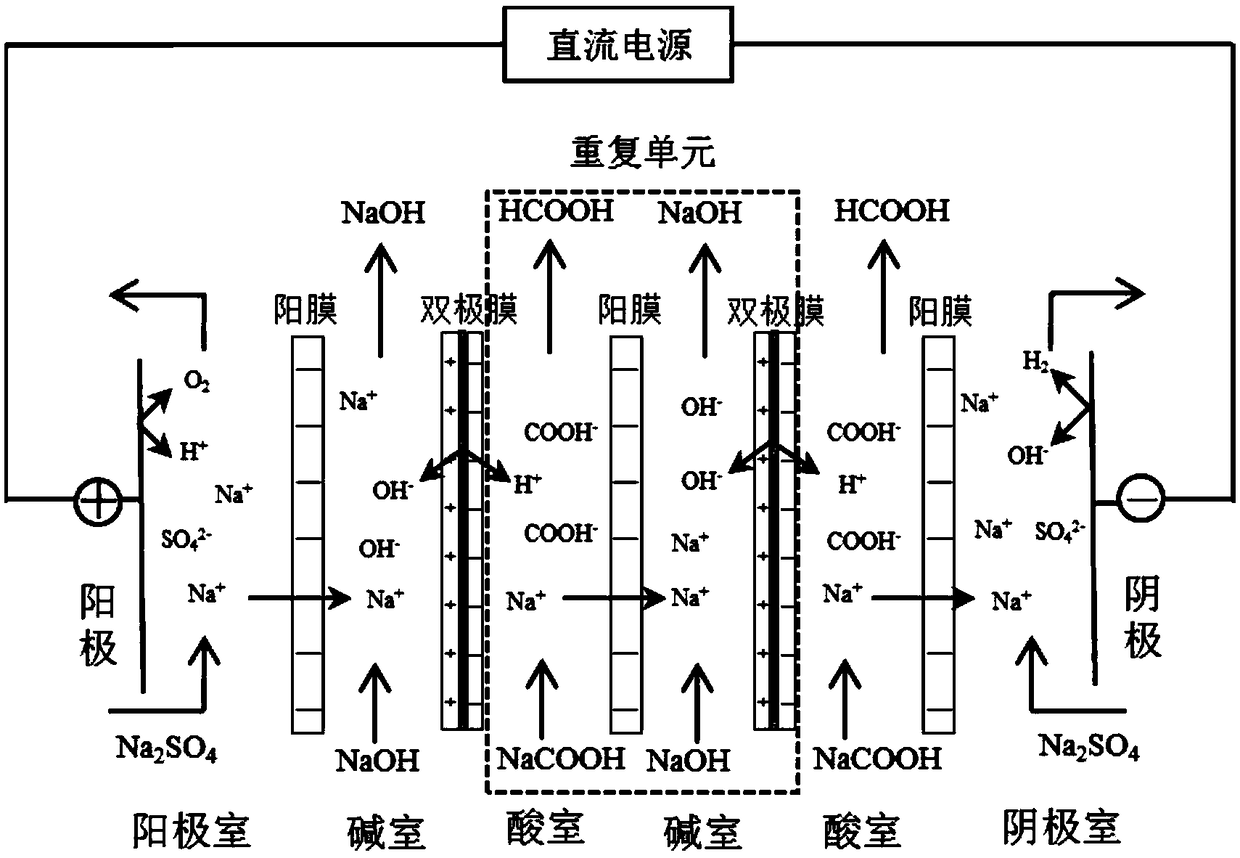
[0020] This embodiment adopts as figure 1 In the bipolar membrane electrodialysis device shown, the arrangement of the membrane stack is anode-anode chamber-cation exchange membrane-alkali chamber-bipolar membrane-[acid chamber-cation exchange membrane-alkali chamber-bipolar membrane] n -acid chamber-cation exchange membrane-cathode chamber-cathode, the number of repeating units n is 5. The material of the anode and cathode in the membrane stack is corrosion-resistant titanium coated with ruthenium. The compartments between adjacent ion exchange membranes include acid chambers and alkali chambers, which are composed of gaskets with flow channels and grids, and the thickness of the gaskets is 0.8mm. The cation exchange membrane used in the membrane stack is CMX produced by Japan Astom Company, the bipolar membrane is BP-1E produced by Japan Astom Company, and the effective area of a single membrane is 189cm 2 (9cm×21cm).
[0021] The anode chamber and the cathode chamber a...
Embodiment 2
[0024] The bipolar membrane electrodialysis device used in this embodiment is the same as that in Embodiment 1.
[0025] The anode chamber and the cathode chamber are connected in series, and 500mL 0.1mol / L Na 2 SO 4 The aqueous solution is used as a strong electrolyte, 500mL of 0.01mol / L NaOH solution is passed into the alkali chamber, and 1000mL of 1mol / L NaCOOH solution is passed into the acid chamber. During the experiment, the linear flow velocity of each compartment solution flowing in the membrane stack was 4cm / s, and the upper limit of the current density was set to 40mA / cm 2 , the upper limit of the membrane stack voltage is set to 20V to avoid damage to the membrane stack under high voltage.
[0026] The experiment runs until the conductivity of the acid chamber no longer decreases, the Na ion content in the acid chamber can be reduced to 0.007mol / L, the conversion rate is 99.3%, and the concentration of formic acid can reach 1.06mol / L at the same time, because the...
Embodiment 3
[0028] The bipolar membrane electrodialysis device used in this embodiment is the same as that in Embodiment 1.
[0029] The anode chamber and the cathode chamber are connected in series, and 500mL 0.1mol / L Na 2 SO 4 The aqueous solution is used as a strong electrolyte, 1000mL 0.01mol / L NaOH solution is passed into the alkali chamber, and 1000mL 3mol / L NaCOOH solution is passed into the acid chamber. During the experiment, the linear flow velocity of each compartment solution flowing in the membrane stack was 4cm / s, and the upper limit of the current density was set to 60mA / cm 2 , the upper limit of the membrane stack voltage is set to 20V to avoid damage to the membrane stack under high voltage.
[0030] The experiment runs until the conductivity of the acid chamber no longer drops, the Na ion content in the acid chamber can be reduced to 0.04mol / L, the conversion rate is 98.7%, and the concentration of formic acid can reach 3.65mol / L at the same time, because the volume of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com