Method for preparing high-purity iron phosphate by utilizing pyrite cinder
A technology of pyrite slag and high-purity phosphoric acid, applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., can solve the problems of low purity of iron phosphate products, low conversion utilization rate of iron elements, etc., to improve the conversion rate. The effect of utilization, mild conditions and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
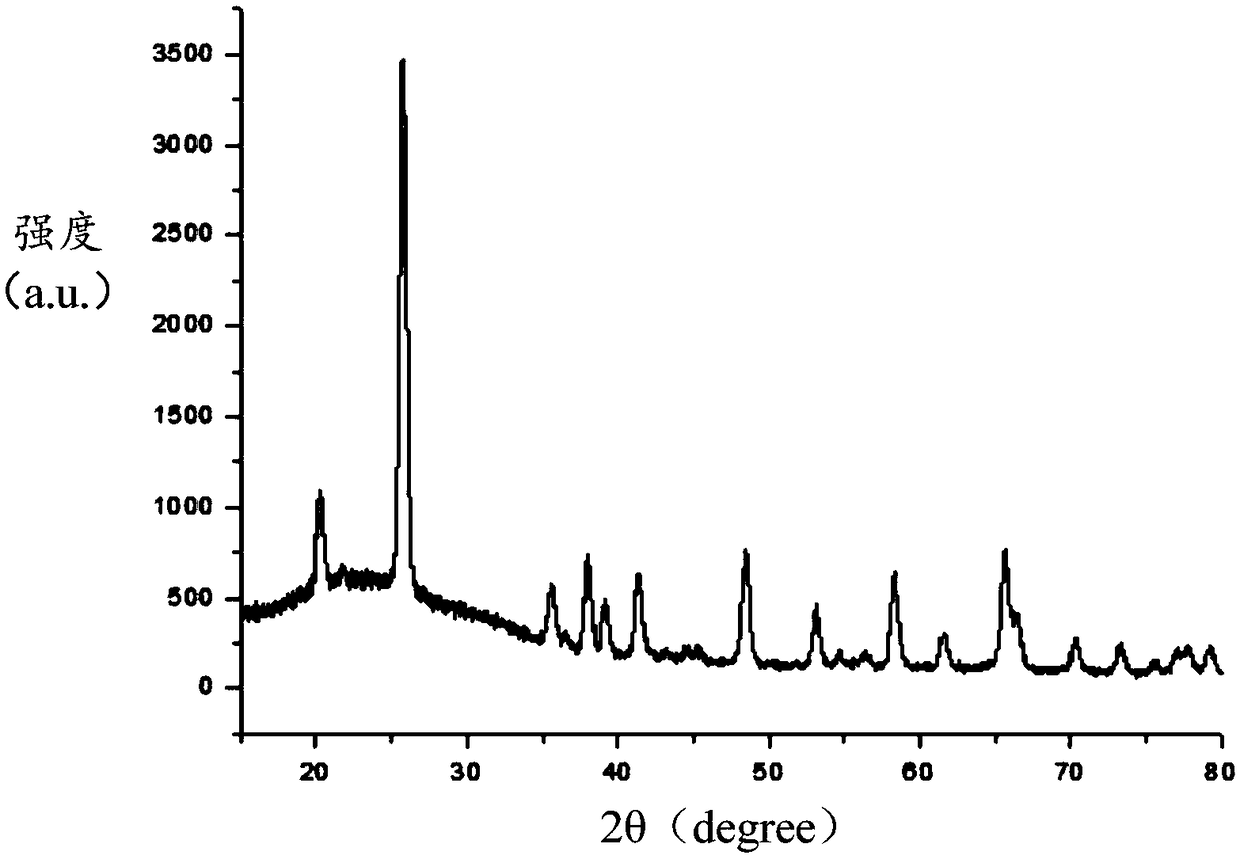
Image
Examples
Embodiment 1
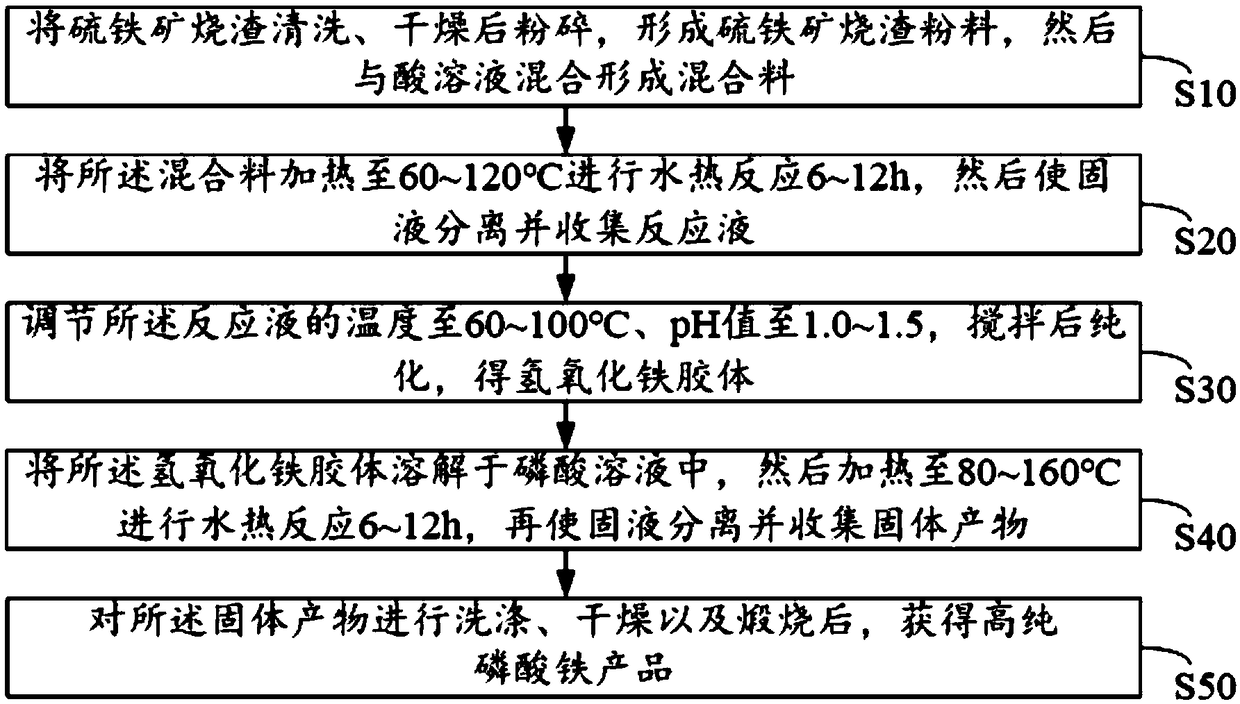
[0065] (1) Pyrite slag (components containing the following mass percentages: 43.31% of ferric oxide, 8.13% of ferric oxide, 1.63% of calcium oxide, 0.46% of magnesium oxide, 35.73% of silicon oxide and 0.16% of sulfur ) washed with water and then dried, then crushed with a high-speed pulverizer and crossed a 200-mesh sieve to obtain a particle size of 200 mesh pyrite slag powder, and then add it to the pyrite slag powder according to the solid-to-liquid ratio of 150g / L Add a sulfuric acid solution with a concentration of 50%, stir until evenly mixed, and form a mixture;
[0066] (2) transfer the mixed material into a hydrothermal reaction kettle, heat to 80° C. for hydrothermal reaction for 8 hours, then filter the reacted mixed material and collect the filtrate to obtain a reaction liquid;
[0067] (3) Add ammonia water to the reaction solution until the pH value is 1.5, then raise the temperature to 100° C., stir for 0.5 h and then transfer to the dialysis bag for purificat...
Embodiment 2
[0072] (1) Pyrite slag (components containing the following mass percentages: 43.31% of ferric oxide, 8.13% of ferric oxide, 1.63% of calcium oxide, 0.46% of magnesium oxide, 35.73% of silicon oxide and 0.16% of sulfur ) washed with water and dried, then crushed with a high-speed pulverizer and crossed a 100-mesh sieve to obtain a particle size of 100 mesh pyrite slag powder, and then add it to the pyrite slag powder according to the solid-to-liquid ratio of 100g / L Add a sulfuric acid solution with a concentration of 40%, stir until evenly mixed, and form a mixture;
[0073] (2) transfer the mixed material into a hydrothermal reaction kettle, heat to 120° C. for hydrothermal reaction for 6 hours, then filter the reacted mixed material and collect the filtrate to obtain a reaction liquid;
[0074] (3) Add ammonia water to the reaction solution until the pH value is 1.0, then raise the temperature to 80°C, stir for 1 hour and then transfer it to the dialysis bag for purification...
Embodiment 3
[0079] (1) Pyrite slag (components containing the following mass percentages: 43.31% of ferric oxide, 8.13% of ferric oxide, 1.63% of calcium oxide, 0.46% of magnesium oxide, 35.73% of silicon oxide and 0.16% of sulfur ) washed with water and dried, then crushed with a high-speed pulverizer and crossed a 500-mesh sieve to obtain a 500-mesh pyrite slag powder, which was then added to the pyrite slag powder according to the solid-to-liquid ratio of 250g / L Add a hydrochloric acid solution with a concentration of 37%, stir until evenly mixed, and form a mixture;
[0080] (2) transfer the mixed material into a hydrothermal reaction kettle, heat to 60° C. for hydrothermal reaction for 12 hours, then filter the reacted mixed material and collect the filtrate to obtain a reaction liquid;
[0081] (3) Add urea to the reaction solution until the pH value is 1.5, then raise the temperature to 100°C, stir for 0.5h, then transfer to the dialysis bag for purification for 18h, and place the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com