Preparation method of anticorrosive coating on surface of electrolytic zinc anode plate
An anti-corrosion coating and anode plate technology, applied in the direction of electrodes, coatings, electrolysis process, etc., can solve the problems of aggravating the corrosion of the anode liquid phase position, reducing the service life of the anode plate, and increasing the lead content of zinc, etc., to achieve good application Benefits, extended service life, and good compactness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Select the lead-silver binary alloy (0.6% silver content) used as the anode plate, and calibrate the position of its liquid level line;
[0028] (2) First carefully wipe the surface of the anode plate with ethanol for 10 minutes, then rinse with water, then wipe the surface with acetone for 15 minutes, and rinse with water;
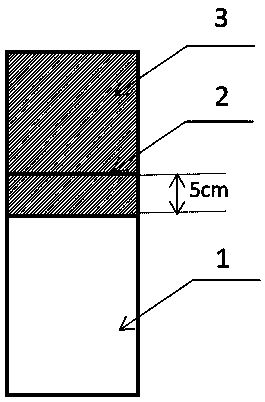
[0029] (3) Delineate the area from the top of the anode plate to 5cm below the liquid level line as the spraying area, and perform sandblasting on the spraying area; the pressure during sandblasting is 0.3MPa-0.5MPa, and the gap between the nozzle and the anode plate surface The angle is 15°-60°, in order to avoid the impact of strong grit on the surface of the anode plate;
[0030] (4) Select NiCoCrAlY powder as the bonding layer material, and zirconia powder with a particle size of 45-75 μm as the spraying material; place NiCoCrAlY powder and zirconia powder in an oven for 5 hours at 80°C;
[0031] (5) Spray a bonding layer with a thickness ...
Embodiment 2
[0035] (1) Select the lead-silver binary alloy (0.6% silver content) used as the anode plate, and calibrate the position of its liquid level line;
[0036] (2) First carefully wipe the surface of the anode plate with ethanol for 10 minutes, then rinse with water, then wipe the surface with acetone for 15 minutes, and rinse with water;
[0037] (3) Delineate the area from the top of the anode plate to 5cm below the liquid level line as the spraying area, and perform sandblasting on the spraying area; the pressure during sandblasting is 0.3MPa-0.5MPa, and the gap between the nozzle and the anode plate surface The angle is 15°-60°, in order to avoid the impact of strong grit on the surface of the anode plate;
[0038] (4) Select Hastelloy C-276 powder as the spraying material, and place the powder in an oven at 80°C for 5 hours;
[0039] (5) Use the plasma spraying method to spray a layer of Hastelloy C-276 coating with a thickness of 150 μm on the sandblasting area after sandbl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com