Method for co-producing and preparing methylthio acetate and diethyl thioglycolate
A technology of thiodiacetate diester and methylthioacetate is applied in directions such as thioether preparation, organic chemistry, etc., can solve problems such as expensive raw materials, high production cost, complicated process, etc., and achieves simple and feasible technological process, The effect of less production of three wastes and high product yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The coproduction method described in this embodiment prepares methyl thioacetate and thiodiacetate diester, comprises the steps:
[0057] (1) Preparation of compounds IV, V, and VI: Add 540 g (3.6 mol) of DL-methionine, 408 g (4.32 mol) of chloroacetic acid, and 3 L of water into a 5 L four-neck flask, stir evenly, slowly heat to 80 ° C, and carry out Reaction for 8 hours; after the reaction, the solvent water was recovered under reduced temperature and reduced pressure to obtain a total of 936 g of DL-homoserine lactone hydrochloride, methylthioacetic acid, and thiodiacetic acid mixture, and directly proceed to the next step without separation;
[0058] (2) Preparation of compound I, II, IV: get the mixture 312g (1.2mol DL-methionine) containing DL-homoserine lactone hydrochloride, methylthioacetic acid, thiodiacetic acid prepared in step (1). , 1.44mol of chloroacetic acid), 300ml of methanol, mix and stir evenly, control the temperature at 30-40°C, add 180.8g (1.52mo...
Embodiment 2
[0061] The coproduction method described in this embodiment prepares methyl thioacetate and thiodiacetate diester, comprises the steps:
[0062] (1) Preparation of compounds IV, V, VI: same as step (1) in Example 1;
[0063] (2) Preparation of compound I, II, IV: 156g (0.6mol DL-methionine, 0.72mol DL-methionine, 0.72mol Chloroacetic acid), 100ml of methanol, stirred evenly at room temperature and filtered, the filter cake was washed with 50ml of methanol; the filtrate was collected and 90.4g (0.76mol) of thionyl chloride was added dropwise to the above filtrate, and the temperature was controlled at 30-40°C. Continue to stir for 2 hours after the addition, cool down to -10°C, filter again, wash the filter cake with methanol (15mL×2 times); combine the two filter cakes and dry to obtain 76.2g of DL-homoserine lactone hydrochloride; the final filtrate Methanol was recovered under reduced pressure, and the remaining liquid was subjected to fractional distillation under reduced ...
Embodiment 3
[0066] The coproduction method described in this embodiment prepares methyl thioacetate and thiodiacetate diester, comprises the steps:
[0067] (1) Preparation of compounds IV, V, VI: same as step (1) in Example 1;
[0068] (2) Preparation of compound I, II, IV: 156g (0.6mol DL-methionine, 0.72mol DL-methionine, 0.72mol Chloroacetic acid), 150ml of ethanol, mix and stir evenly, control the temperature at 30-40°C, add 90.4g (0.76mol) of thionyl chloride dropwise, continue stirring for 2h after adding, cool down to -10°C, filter, and use ethanol (30mL×2 times) wash the filter cake, and dry to obtain 75.0g of DL-homoserine lactone hydrochloride; the filtrate reclaims ethanol under reduced pressure, and the remaining liquid is fractionated under reduced pressure to obtain 58.9g of ethyl methylthioacetate (content 98.5%), diethyl thiodiacetate 18.6g (content 98.3%).
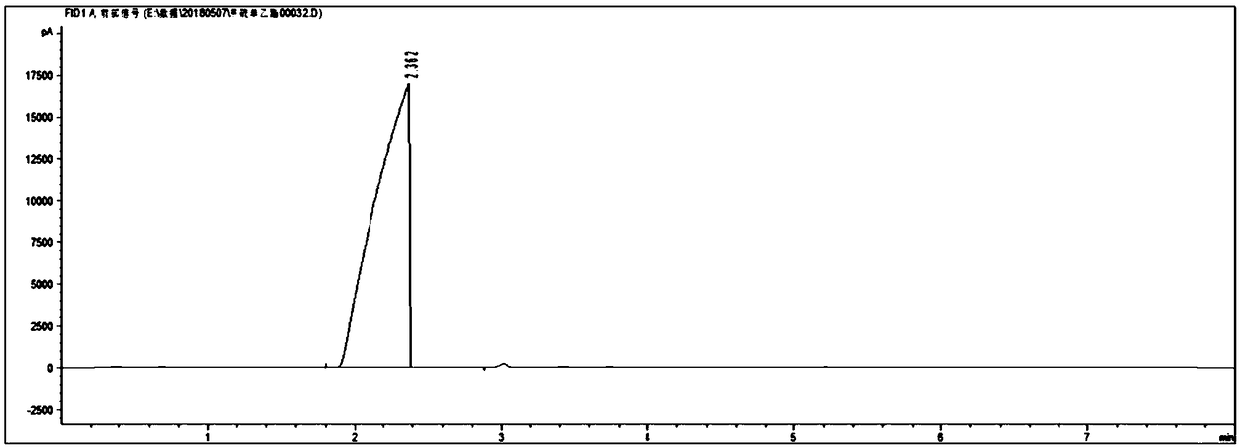
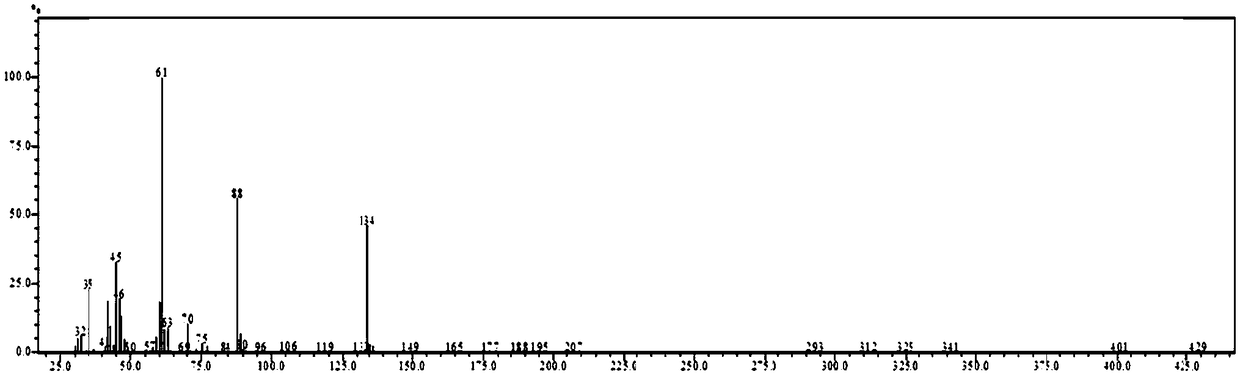
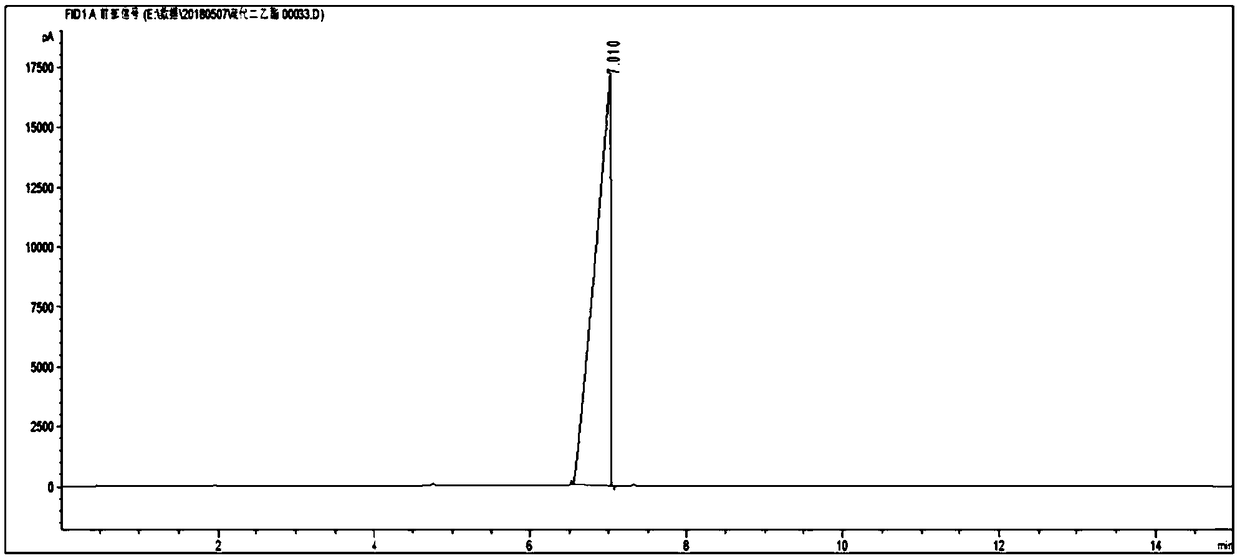
[0069] Detect according to the method in the prior art, the gas chromatogram and the mass spectrogram of gained ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com