Drug for preventing and treating coronary heart diseases and preparation method of drug
A drug and pharmacy technology, applied in the field of medicinal chemistry, can solve the problem of ineffective treatment of coronary heart disease, etc., and achieve the effect of excellent LCAT activation effect, increase HDL level, and excellent activation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
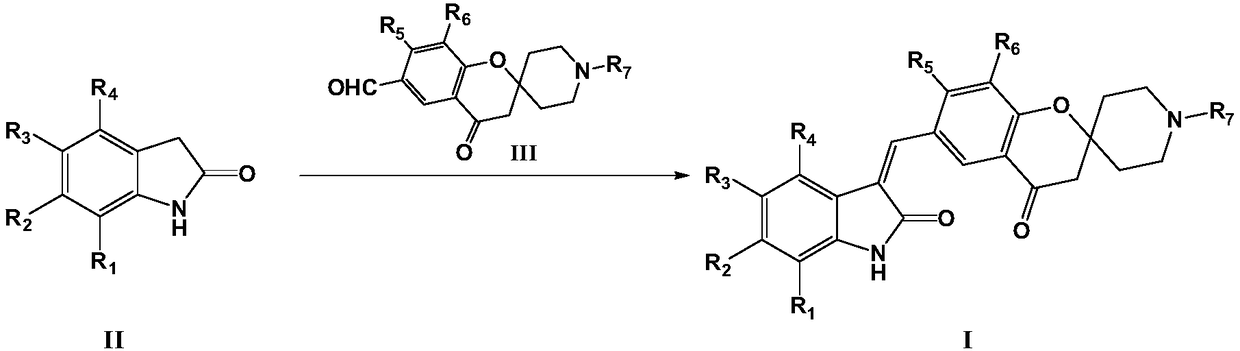
[0045] The present invention also provides a preparation method of the compound of formula I, the preparation method comprising the following steps:
[0046] Reaction of a compound of formula II with a compound of formula III in the presence of a base to prepare a compound of formula I
[0047]
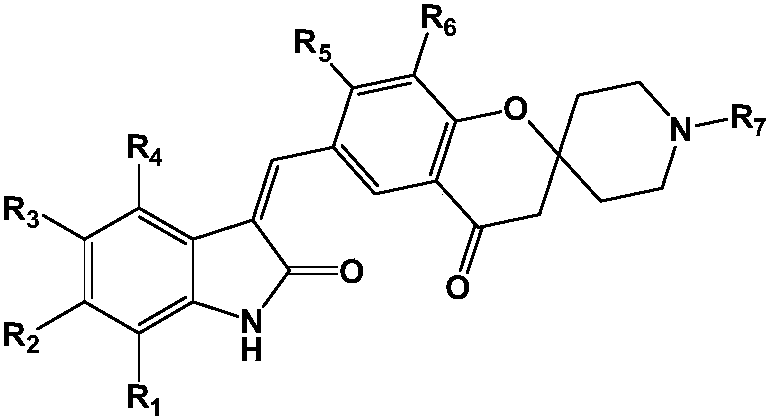
[0048] Among them, R 1 -R 7 as defined herein;
[0049] The base is selected from organic bases such as triethylamine or pyridine; or inorganic bases such as sodium hydroxide, potassium hydroxide, sodium carbonate or potassium carbonate.
[0050] The present invention also relates to a pharmaceutical composition comprising at least one of said compounds or pharmaceutically acceptable salts, stereoisomers, tautomers, solvates, and prodrugs thereof as an active ingredient. The pharmaceutical composition may comprise a pharmaceutically acceptable carrier or excipient.
[0051] The pharmaceutical composition can be prepared according to methods known in the art. Any dosage form su...
Embodiment 1
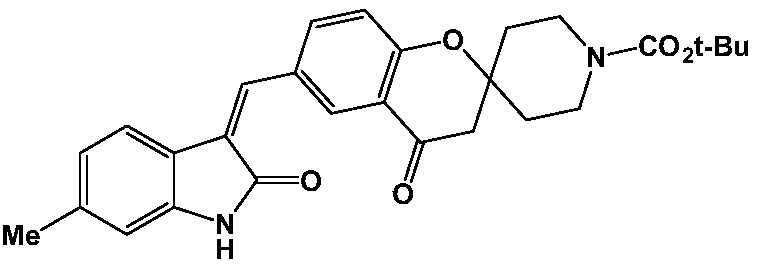
[0062] Example 1: (Z)-6-((6-methyl-2-oxoindole-3-methylene)methyl)-4-oxospiro[chroman-2,4'-piperidine ]-1'-tert-butyl formate (compound SP-1)
[0063]
[0064] Put tert-butyl 6-formyl-4-oxospiro[chroman-2,4'-piperidine]-1'-carboxylate (10.0mmol) into the flask, add 100m absolute ethanol to dissolve it, and then Then 6-methylindolin-2-one (10.0 mmol) and 2 ml of triethylamine were added, and the reaction was stirred at room temperature for 3 h, and a large amount of precipitation appeared. Suction filtration under reduced pressure, the filter cake was washed with a small amount of absolute ethanol, and dried under vacuum to obtain 4.34 g of the title product as a white solid, with a yield of 91.5%.
[0065] ESI-MS: 475.22[M+H] +
[0066] Elemental analysis: theoretical value C, 70.87; H, 6.37; N, 5.90; O, 16.86
[0067] Found value C, 70.58; H, 6.55; N, 5.79; O, 17.08
[0068] 1 H NMR (400MHz, CDCl 3 )δ11.08(s,1H),7.85(d,1H),7.70(s,1H),7.51(d,1H),7.39(d,1H),7.13(s,1H)...
Embodiment 2
[0069] Example 2: (Z)-6-((5,6-dimethoxy-2-oxoindoline-3-methylene)methyl)spiro[chroman-2,4'-piperidine ]-4-one (SP-2)
[0070]
[0071] According to the method of Example 1, replace 6-formyl-4-oxospiro[chroman-2,4'-piperidine]-6-carbaldehyde with 4-oxospiro[chroman-2,4'-piperidine Pyridine]-1'-carboxylic acid tert-butyl ester, substituting 5,6-dimethoxyindolin-2-one for 6-methylindolin-2-one afforded the title compound as a gray solid, yield 87.9 %.
[0072] ESI-MS: 423.18[M+H] +
[0073] Elemental analysis: theoretical value C, 68.23; H, 6.20; N, 6.63; O, 18.94
[0074] Found value C, 68.35; H, 6.02; N, 6.97; O, 18.66
[0075] 1 H NMR (400MHz, CDCl 3 )δ11.10(s,1H),7.88(d,1H),7.53(s,1H),7.29(s,1H),7.22(s,1H),7.10(d,1H),6.89(d,1H ), 3.85(s,6H), 2.85(t,4H), 2.66(s,2H), 2.02(s,1H), 1.91(t,4H).
[0076] According to a method similar to Example 1, the following compounds were synthesized:
[0077]
[0078] Example of efficacy
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com