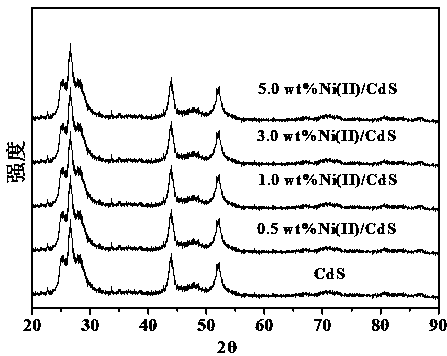
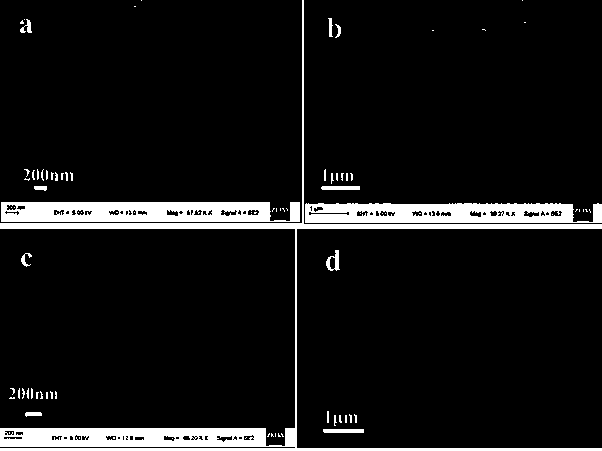
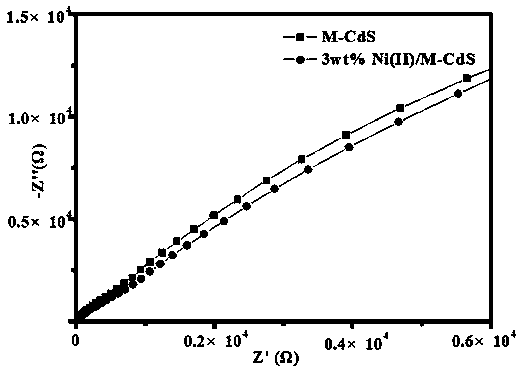
Preparation of nickel (II) supported mixed phase cadmium sulfide material and application of photocatalytic activation saturated hydrocarbon bonds
A cadmium sulfide, mixed-phase technology, used in the preparation of organic compounds, carbon-based compounds, catalysts for physical/chemical processes, etc., can solve the problems of low toluene conversion, low efficiency, and low speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (1) Preparation of CdS: Add cadmium nitrate into deionized water at a ratio of 0.5~5.0mmol: 2.0~20.0mL, stir and dissolve completely at 18~25°C to obtain cadmium nitrate aqueous solution; add 0.04ml oleic acid to it, that is Obtain A solution; then add sodium borohydride into deionized water at a ratio of 2.0~15.0mmol:0.4~3.0mL, stir and dissolve completely at 18~25°C to obtain sodium borohydride aqueous solution; then add it to the A solution , mixed evenly and treated by microwave, the mixed solution changed from colorless solution to black solution, cooled to room temperature; adding 1 to 3 times the volume of absolute ethanol to the black solution to wash, centrifuged 3 to 5 times to obtain black Solid; ultrasonically disperse the black solid in absolute ethanol at a ratio of 0.5~5.0mol:2~20mL, add ammonium sulfide solution after magnetic stirring for 10~15min, and stir at 18~25°C for 0.5~3h to obtain yellow or Yellow-green solution; centrifuge with absolute ethanol...
Embodiment 2
[0029] (1) Preparation of CdS: Same as Example 1;
[0030] (2) Preparation of Ni(II) / CdS: Take 200mg of CdS prepared in step (1) and evenly disperse in 10ml of deionized water; take 8.10mg of nickel chloride and disperse it in 10ml of deionized water to dissolve; then mix the above two solutions In a 25 mL flask, stirred at 70°C for 1.5h, the reaction solution was washed three times with deionized water, and the resulting product was dried in an oven at 60°C for 4h to obtain Ni(II) with a Ni(II) loading of 1.0wt%. ) / CdS samples. The sample was used to catalyze the oxidation of toluene to benzaldehyde at a rate of 189.08 μmolh -1 g -1 ), the conversion rate of toluene was 8.3%; the selectivity for the activation of saturated C-H (sp3) bond in toluene was up to 72.9%.
Embodiment 3
[0032] (1) Preparation of CdS: Same as Example 1;
[0033] (2) Preparation of Ni(II) / CdS: take 200mg of CdS prepared in step (1) and evenly disperse in 10ml of deionized water; take 24.30mg of nickel chloride and disperse it in 10ml of deionized water to dissolve; then mix the above two solutions In a 25 mL flask, and stirred at 80°C for 2h, the reaction solution was washed three times with deionized water, and the resulting product was dried in an oven at 60°C for 4h to obtain Ni(II) with a Ni(II) loading of 3.0wt%. / CdS sample. The sample was used to catalyze the oxidation of toluene to benzaldehyde at a rate of 216.70 μmolh -1 g -1 ), the conversion rate of toluene was 8.8%; the selectivity for the activation of saturated C-H (sp3) bond in toluene was up to 78.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com