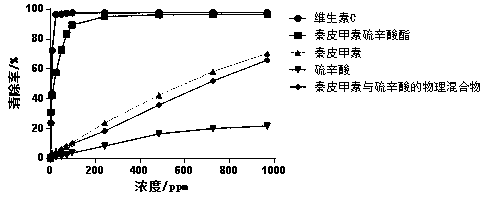
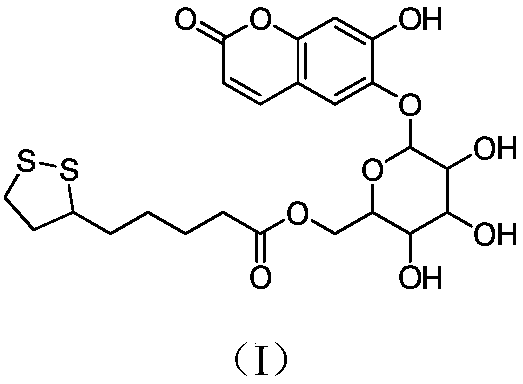
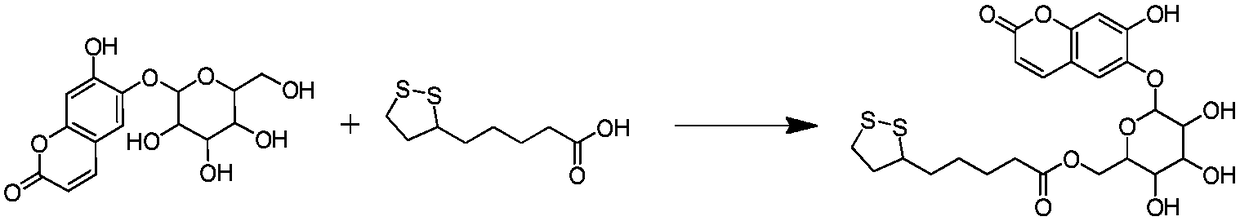
Esculin hydrate thioctic ester compound with antioxidant activity, and preparation method thereof
An anti-oxidant activity, the technology of echinocortin, is applied in the direction of food ingredients containing organic compounds, food ingredients as antioxidants, organic chemistry, etc., to achieve the effect of preventing oxidative discoloration, easy availability of raw materials, and high utilization of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0024] Put 100mg (0.29mmol) afraetin and 66.6mg (0.32mmol) lipoic acid in a 100mL reactor, add 60mL acetonitrile to fully dissolve, add 61.9mg (0.3mmol) DCC and 3.5mg chlorinated chlorinated Sulfone was protected by nitrogen gas. After ultrasonic reaction at 35°C for 4 hours, the obtained reaction liquid was cooled to room temperature, concentrated under reduced pressure, and left to separate layers. The lower oil phase was separated by column chromatography, and the eluent was anhydrous sodium sulfate After drying, filtering, and concentrating under reduced pressure, 137.9 mg of the target product acridine lipoate was obtained, with a yield of 88.79%.
[0025] 1 H-NMR (400MHz, DMSO-d6) δ (ppm): 7.74 (1H, d), 6.87 (1H, s), 6.73 (1H, s), 5.90 (1H, d), 5.81 (1H, d), 5.31(1H, s), 4.01-4.30(3H, m), 3.89(1H, t), 3.52(3H, s), 3.39-3.50(2H, m), 2.63-2.49(3H, m), 2.30( 2H, t), 2.00-1.71 (4H, m), 1.53 (2H, q), 1.28 (2H, m); 13 C-NMR (400MHz, DMSO-d 6 )δ (ppm): 173.4, 160.6, 148.6, ...
preparation Embodiment 2
[0028] Put 100mg (0.29mmol) afracetin and 60.6mg (0.29mmol) lipoic acid in a 100mL reactor, add 60mL acetonitrile to fully dissolve, add 61.9mg (0.3mmol) DCC and 6.7mgTEBAC to the reactor, and feed Nitrogen protection, ultrasonic reaction at 45°C for 4h, cooling the obtained reaction liquid to room temperature, concentrating under reduced pressure, standing for layering, taking the lower layer of oil phase column chromatography, and drying and filtering the eluent with anhydrous sodium sulfate, reducing Concentrated under reduced pressure to obtain 121.3 mg of the target product afraxin lipoate, with a yield of 78.14%.
preparation Embodiment 3
[0031] Put 100mg (0.29mmol) alopecia and 78.7mg (0.38mmol) lipoic acid in a 100mL reactor, add 60mLTHF to fully dissolve, add 61.9mg (0.3mmol) DCC and 3.5mg thionyl chloride to the reactor , pass through nitrogen protection, after ultrasonic reaction at 55°C for 3 hours, cool the obtained reaction solution to room temperature, concentrate under reduced pressure, stand and separate layers, take the lower oil phase and separate it by column chromatography, and dry the eluent with anhydrous sodium sulfate Filtration and concentration under reduced pressure gave 129.3 mg of the target product, afracetin lipoate, with a yield of 83.26%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com