Bidentate phosphine ligand and application thereof in hydroformylation reaction
A technology for bidentate phosphine ligands and oxidation reactions, which is applied in the field of bidentate phosphine ligands and their application in hydroformylation reactions, can solve the problem of low linear selectivity of triphenylphosphine ligands, and achieve good results. Effects of catalytic activity and linear selectivity, simple structure, and strong chelating ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
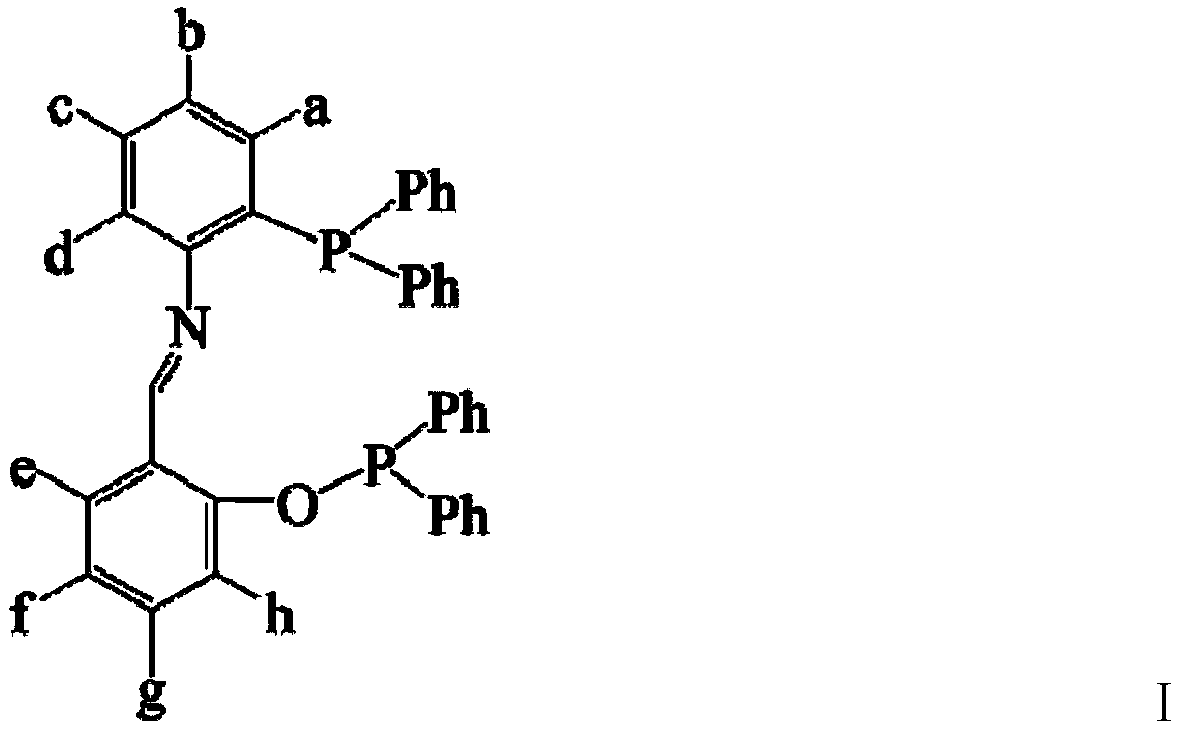
[0027] A bidentate phosphine ligand A having the following structure and a preparation method thereof:
[0028]
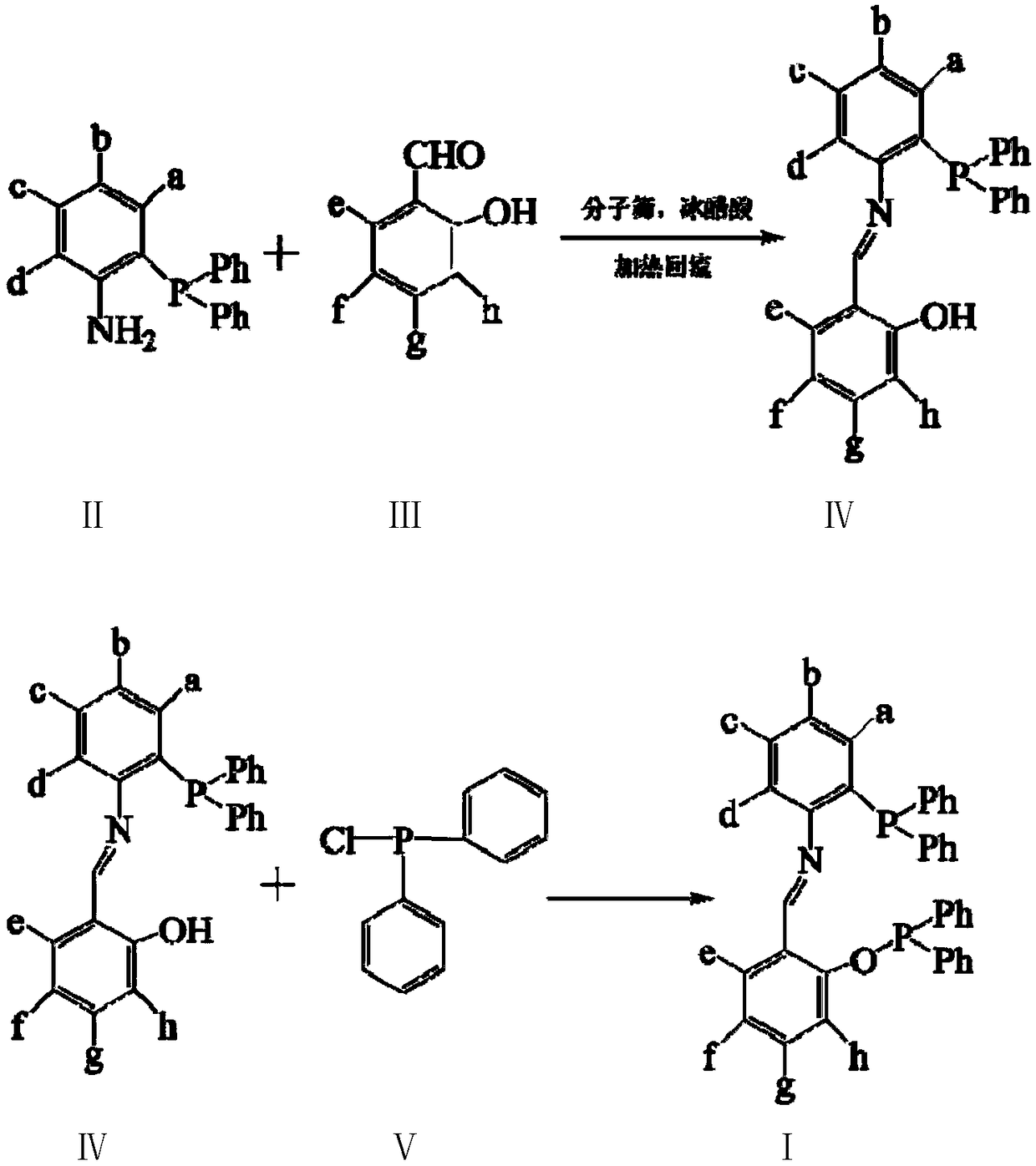
[0029] Add 1mL (9.6mmol) of salicylaldehyde and 3.4g (9.6mmol) of 3-diphenylphosphino-4-aminobiphenyl, 30ml of absolute ethanol, molecular sieves and glacial acetic acid in the reaction flask, heat and reflux for 24 hours, remove Molecular sieves, concentrated, cooled to room temperature to obtain crude product, recrystallized with ethanol to obtain light yellow crystal 2.8g, namely intermediate product A1 (63.8%); add 30ml absolute ethanol, 1.1mL (6.1mmol) diphenyl Add 2.8gA1 (6.1mmol) after phosphorus chloride is mixed evenly, react at room temperature for 3 hours, concentrate, cool and crystallize to obtain a crude product, recrystallize with ethanol to obtain light yellow crystals, namely bidentate phosphine ligand A3.1g (79.2%) .
[0030] The elemental analysis of the bidentate phosphine ligand A: actual measurement (calculated value): C: 80.47 (80.5); H: ...
Embodiment 2
[0032] A bidentate phosphine ligand B having the following structure and a preparation method thereof:
[0033]
[0034] In the reaction flask, add 0.5mL (4.8mmol) salicylaldehyde and 1.9g (4.8mmol) 3-diphenylphosphino-4-amino-3'nitrobiphenyl, 20ml absolute ethanol, molecular sieves and glacial acetic acid, Heat to reflux for 24 hours, remove molecular sieves, concentrate, cool to room temperature to obtain crude product, recrystallize with ethanol to obtain light yellow crystal 1.4g, i.e. intermediate product B1 (58.1%); add 20ml absolute ethanol in reaction flask, 0.5mL ( 2.8mmol) of diphenylphosphine chloride was mixed evenly, then added 1.4g B1 (2.8mmol), reacted at room temperature for 3 hours, concentrated, cooled and crystallized to obtain a crude product, recrystallized with ethanol to obtain light yellow crystals, namely the bidentate phosphine ligand B1 .4g (72.9%).
[0035] The elemental analysis of the bidentate phosphine ligand B: actual measurement (calculate...
Embodiment 3
[0037] A bidentate phosphine ligand C having the following structure and a preparation method thereof:
[0038]
[0039]Add 2.1g (9.9mmol) 3-phenyl-5-methyl salicylaldehyde and 2.8g (10.1mmol) (o-aminophenyl) diphenylphosphine in the reaction flask, 50ml absolute ethanol, molecular sieves and glacial acetic acid , heated to reflux for 24 hours, removed molecular sieves, concentrated, cooled to room temperature to obtain crude product, recrystallized with ethanol to obtain light yellow crystal 3.3g, namely intermediate product C1 (70.8%); add 30ml absolute ethanol in reaction flask, 0.9mL (5mmol) diphenylphosphine chloride was mixed evenly, then added 2.4gC1 (5.1mmol), reacted at room temperature for 3 hours, concentrated, cooled and crystallized to obtain a crude product, recrystallized with ethanol to obtain light yellow crystals, namely the bidentate phosphine ligand C2 .5 g (76.3%).
[0040] The elemental analysis of the bidentate phosphine ligand C: measured (calculate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com