Cadmium ion adsorbent and preparation method thereof
A cadmium ion and adsorbent technology, applied in the field of cadmium ion adsorbent and its preparation, achieves the effects of stable adsorption performance, broad industrial application prospect, high adsorption rate and adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: synthetic cadmium ion adsorbent
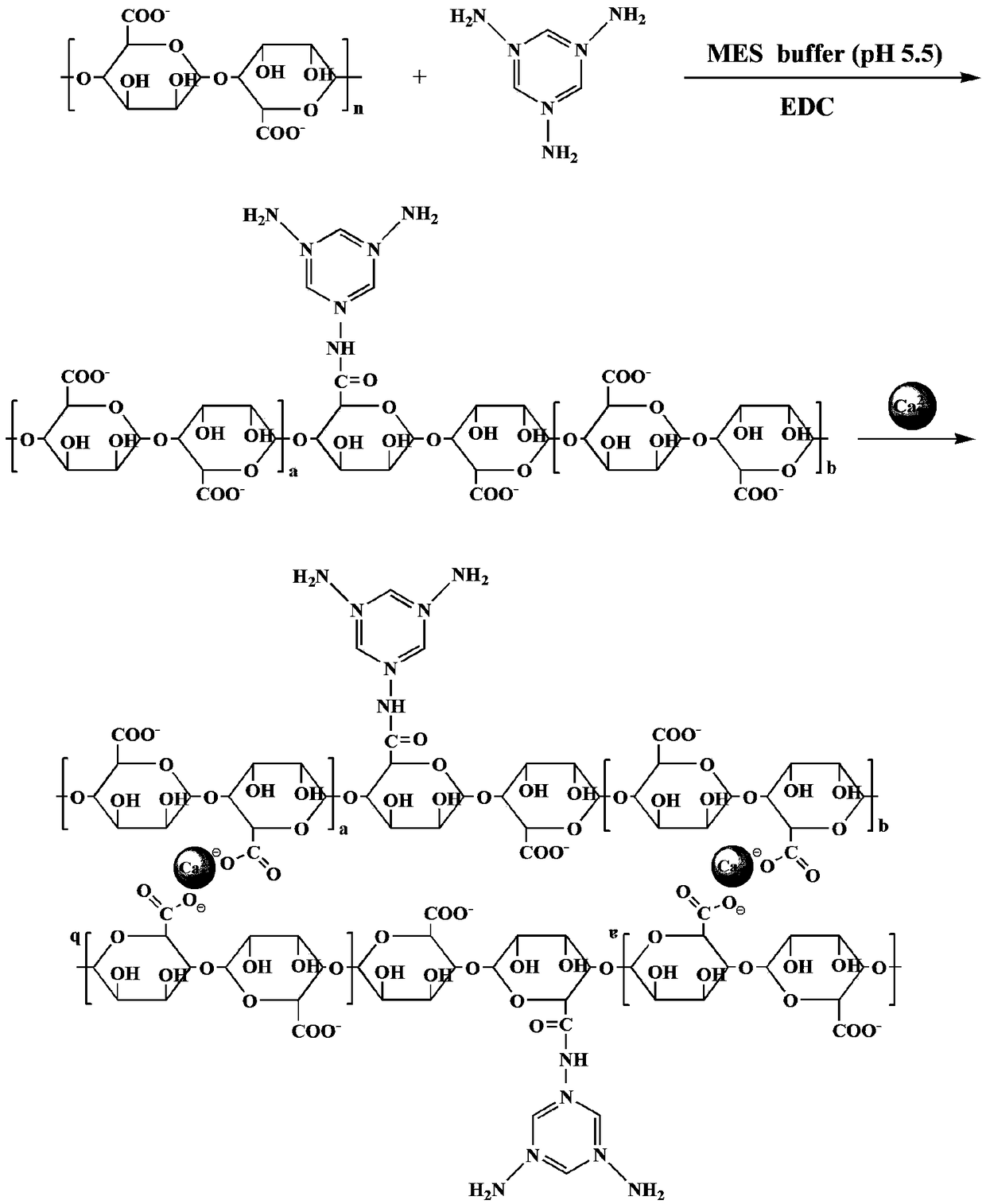
[0025] figure 1 Prepare the synthetic route map of cadmium ion adsorbent for this embodiment, as figure 1 As shown, the specific steps are as follows:
[0026] (1) Weigh 1.5g sodium alginate (viscosity: 200±20mPa·s) and dissolve it in 98.5g pH5.5 2-morpholineethanesulfonic acid-sodium hydroxide (MES) buffer solution, and add 0.15g 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and 0.5g melamine, under the condition of 25 ℃, continue to stir and react for 12h, and then use a 10mL medical syringe to Add the mixed solution dropwise to 500mL 0.2mol / L calcium nitrate solution, continue to stir for 20min after dropping, filter and wash with distilled water to prepare calcium alginate-melamine composite hydrogel;
[0027] (2) Soak the calcium alginate-melamine composite hydrogel prepared in step (1) in distilled water, freeze and vacuum-dry in a vacuum freeze dryer, (the condition of vacuum freeze-drying is:...
Embodiment 2
[0028] Embodiment 2: synthetic cadmium ion adsorbent
[0029] (1) Weigh 1.5g potassium alginate and dissolve it in 98.5g pH 5.5 2-morpholineethanesulfonic acid (MES) buffer solution, and add 0.15g 1-(3-dimethylaminopropyl)- 3-Ethylcarbodiimide hydrochloride (EDC) and 0.5g melamine, under the condition of 25 ℃, continue to stir and react for 12h, and then use a 10mL medical syringe to drop the mixed solution into 500mL0.2mol / L calcium nitrate solution In the process, continue to stir for 20 minutes after dropping, filter and wash with distilled water to obtain calcium alginate-melamine composite hydrogel;
[0030] (2) Soak the calcium alginate-melamine composite hydrogel prepared in step (1) in distilled water, place in a vacuum freeze dryer to freeze and dry in vacuum, and make calcium alginate-melamine composite aerogel (cadmium ion adsorbent).
[0031] The difference between Example 2 and Example 1 is that sodium alginate is replaced by potassium alginate.
Embodiment 3
[0032] Embodiment 3: synthetic cadmium ion adsorbent
[0033] (1) Weigh 1.5g of alginic acid and dissolve it in 98.5g of 2-morpholineethanesulfonic acid-sodium hydroxide (MES) buffer solution with pH 5.5, and add 0.15g of 1-(3-dimethylaminopropyl )-3-Ethylcarbodiimide hydrochloride (EDC) and 0.5g melamine, under the condition of 25 ℃, continue to stir and react for 12h, and then use a 10mL medical syringe to drop the mixed solution into 500mL0.2mol / L nitric acid In the calcium solution, continue to stir for 20 minutes after dropping, filter and wash with distilled water to obtain calcium alginate-melamine composite hydrogel;
[0034] (2) Soak the calcium alginate-melamine composite hydrogel prepared in step (1) in distilled water, place in a vacuum freeze dryer to freeze and dry in vacuum, and make calcium alginate-melamine composite aerogel (cadmium ion adsorbent).
[0035] The difference between Example 3 and Example 1 is that sodium alginate is replaced by alginic acid. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com