A kind of synthetic method of avibactam intermediate
A synthesis method and a technology for intermediates, applied in the field of medicinal chemistry synthesis, can solve problems such as affecting product quality and yield, easy ignition of sodium hydride, strong alkali activity, etc., and achieve carbonate safety, less by-products, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036]
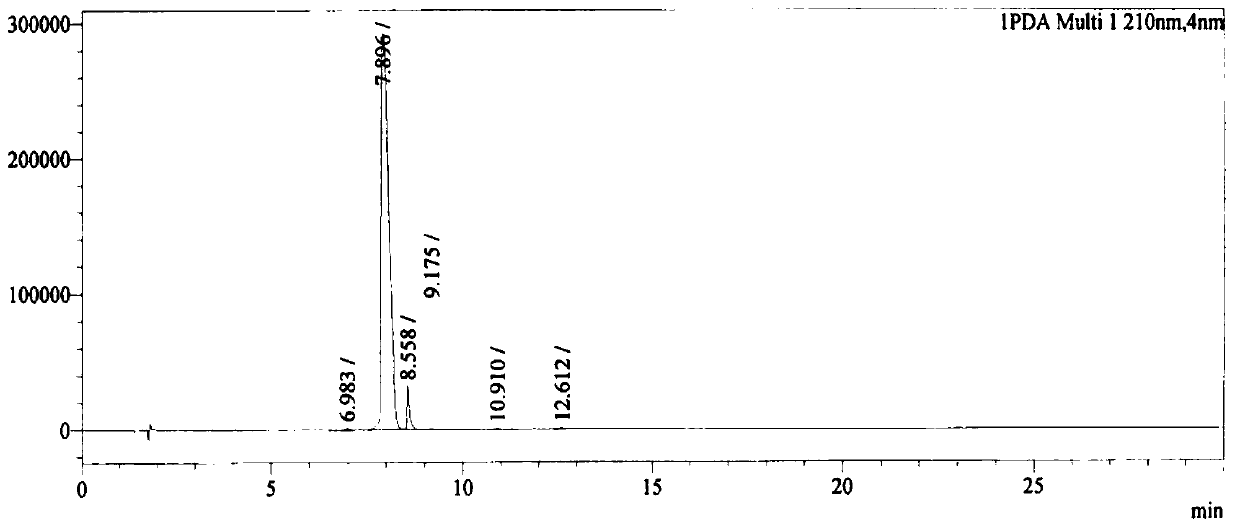
[0037] In a 3L three-necked flask, add 900mL dimethylsulfoxide, 55.2g (2.0eq, 250.9mmol) trimethylsulfoxide iodide, 70.0g (4.0eq, 506.9mmol) potassium carbonate and 40.0g (1.0eq, 125.4 mmol) N-Boc-benzyl pyroglutamate, heated to 50°C for 23h. After that, the heating was stopped, and 500 mL of saturated ammonium chloride aqueous solution was added dropwise. After the dropwise addition, 500 mL of purified water was added for dilution, and extracted with ethyl acetate (500 mL×2). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 49.5 g of a light yellow solid product with a yield of 96% and a purity of 96.0%. figure 1 , see Table 2 for liquid chromatography data, ESI-MS m / z: 412.2 ([M+H] + ).
[0038] The liquid phase spectrogram data of table 2 embodiment 1 product
[0039]
Embodiment 2
[0041] In a 3L three-necked flask, add 1000mL dimethylsulfoxide, 50.0 (1.8eq, 227.3mmol) trimethylsulfoxide iodide, 61.0g (3.5eq, 441.7mmol) potassium carbonate, 40.0g (1.0eq, 125.4mmol) N-Boc-benzyl pyroglutamate, heated to 45°C for 20h. Heating was stopped, and 500 mL of saturated aqueous ammonium chloride was added dropwise, followed by 500 mL of purified water for dilution, and extracted with ethyl acetate (500 mL×2). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 47.4 g of a yellow solid product with a yield of 92% and a purity of 94.9%.
Embodiment 3
[0043]In a 3L three-necked flask, add 900mL dimethylsulfoxide, 55.2g (2.0eq, 250.9mmol) trimethylsulfoxide iodide, 78.0g (4.5eq, 564.8mmol) potassium carbonate, 40.0g (1.0eq, 125.4 mmol) N-Boc-benzyl pyroglutamate, heated to 55°C for 25h. Heating was stopped, and 500 mL of saturated aqueous ammonium chloride was added dropwise, followed by 500 mL of purified water for dilution, and extracted with ethyl acetate (500 mL×2). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated under reduced pressure to obtain 49.1 g of a yellow solid product with a yield of 95% and a purity of 95.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com