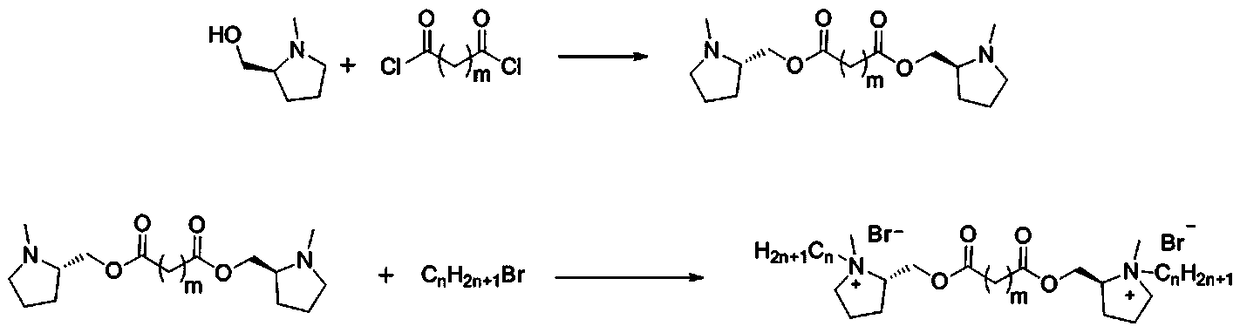
Pyrrolidine type chiral Gemini surfactant with linking groups containing ester groups and preparation method thereof
A technology of surfactant and pyrrolidine, which is applied in the field of pyrrolidine chiral Gemini surfactant and its preparation, can solve the problems of synthesis and performance vacancy, and achieve the effect of easy separation and purification, easy degradation, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The structural formula of surfactant (I) is as follows:
[0043] .
[0044] The preparation method of surfactant (I) comprises the steps:
[0045] The first step, the synthesis of bis(N-methyl-L-prolinol) adipate
[0046] Add 9.30 g (80.74 mmol) of N-methyl-L-prolinol, 50 mL of dichloromethane and 12.26 g (121.11 mmol) of triethylamine into a 250 mL three-neck flask, blow in nitrogen, and cool to 0 in an ice bath. ~10°C. Slowly add 7.39 g (40.37 mmol) of adipoyl chloride dropwise, and react for 8 h after the addition of adipyl chloride is complete. After the reaction was over, 100 mL of saturated sodium carbonate was added, and the mixture was stirred for 3 min to separate the layers. The organic layer was washed with water, dried with an appropriate amount of anhydrous magnesium sulfate, decolorized with activated carbon, and rotary evaporated to obtain a colorless viscous liquid, namely bis(N-methyl-L-prolinol) adipate.
[0047] The second step, the synthesis o...
Embodiment 2
[0051] The structural formula of surfactant (II) is as follows:
[0052] .
[0053] The preparation method of surfactant (II) comprises the steps:
[0054] The first step, the synthesis of bis(N-methyl-L-prolinol) adipate
[0055] Add 9.30g (80.74 mmol) of N-methyl-L-prolinol, 80mL of chloroform and 25.68g (242.22mmol) of anhydrous sodium carbonate into a 250mL three-neck flask, blow in nitrogen, and cool in an ice bath to 0~ 10°C. Slowly add 7.39 g (40.37 mmol) of adipoyl chloride dropwise, and react for 6 hours after the addition of adipoyl chloride is complete. After the reaction was over, 100 mL of saturated sodium carbonate was added, and the mixture was stirred for 3 min to separate the layers. The organic layer was washed with water, dried with an appropriate amount of anhydrous magnesium sulfate, decolorized with activated carbon, and rotary evaporated to obtain a colorless viscous liquid, namely bis(N-methyl-L-prolinol) adipate.
[0056] The second step, the sy...
Embodiment 3
[0060] The structural formula of surfactant (III) is as follows:
[0061] .
[0062] The preparation method of surfactant (III) comprises the steps:
[0063] The first step, the synthesis of bis(N-methyl-L-prolinol) succinate
[0064] Add 9.30 g (80.74 mmol) of N-methyl-L-prolinol, 100 mL of anhydrous acetone and 25.55 g (322.96 mmol) of pyridine into a 250 mL three-neck flask, blow in nitrogen gas, and cool in an ice bath to 0-10°C . Slowly add 6.26 g (40.37 mmol) of succinoyl chloride dropwise, and react for 3 h after the addition of succinoyl chloride is complete. After the reaction was completed, 100 mL of saturated sodium carbonate was added, and the mixture was stirred for 3 min to separate the layers. The organic layer was washed with water, dried with anhydrous magnesium sulfate, decolorized with activated carbon, and rotary evaporated to obtain a colorless viscous liquid, that is, bis(N-methyl-L-prolinol) succinate.
[0065] The second step, the synthesis of su...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com