3-acylamino-N-arylbenzamide compound as well as preparation and application thereof
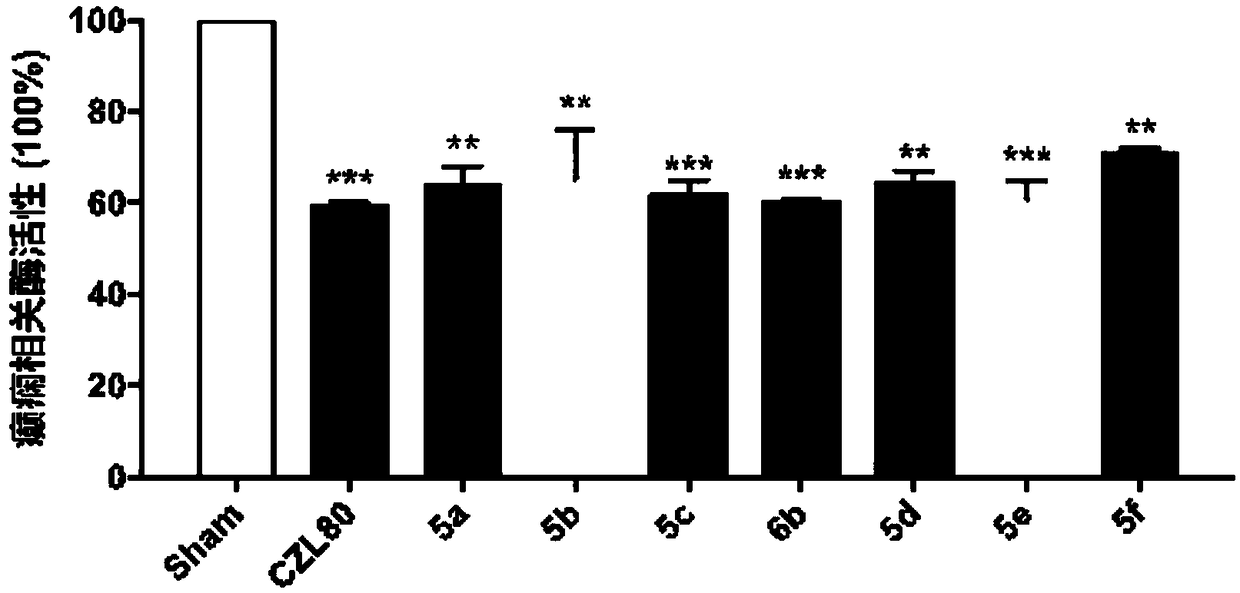
An arylbenzamide and amide technology, applied in the field of medicine, can solve problems such as sequelae, and achieve the effect of raising the seizure threshold, improving the therapeutic effect, and reducing the level of epileptic seizures and mortality.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
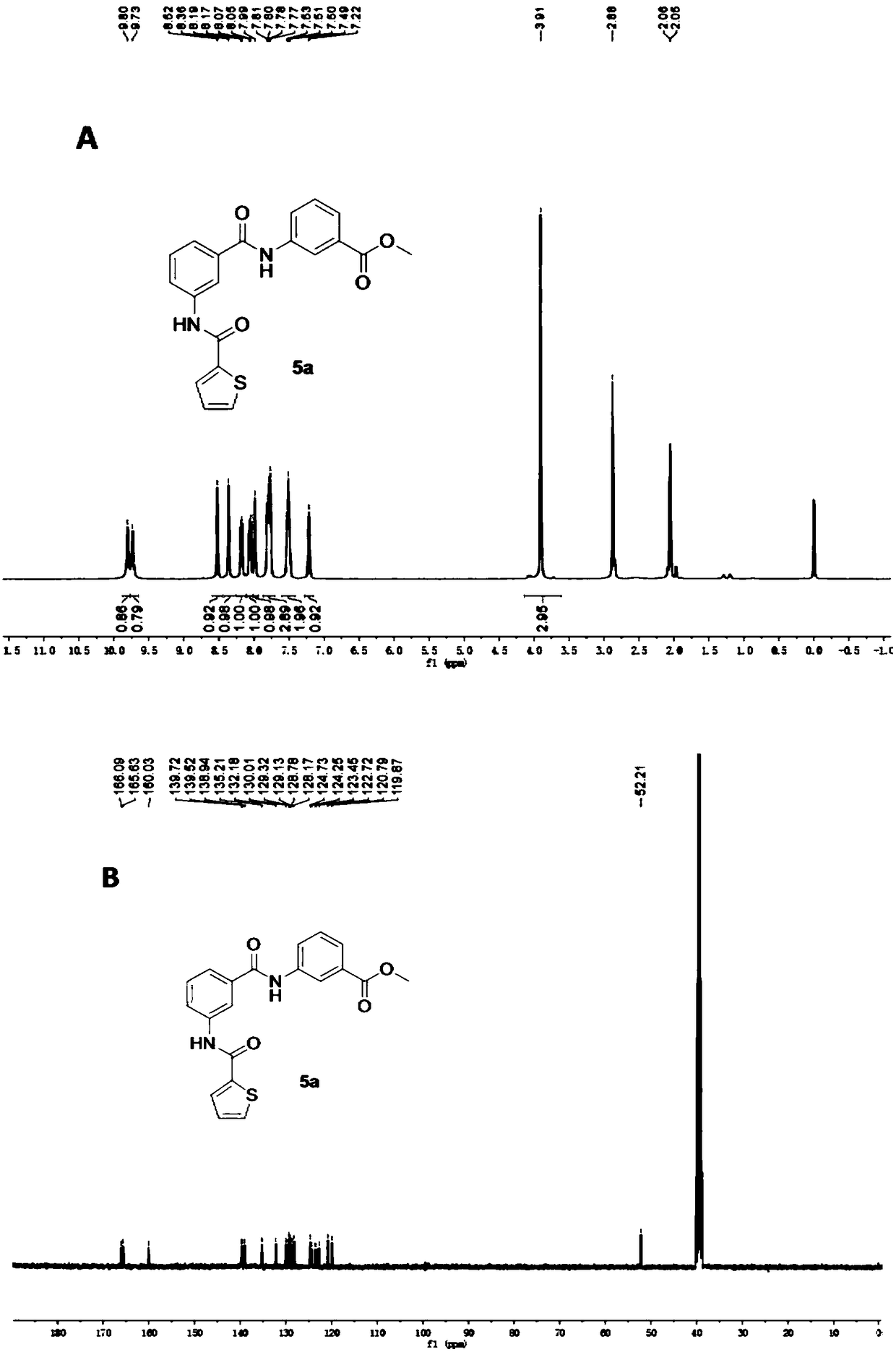
[0048] Example 1 3-(3-(thiophene-2-carboxamido)benzamido)methyl benzoate
[0049]
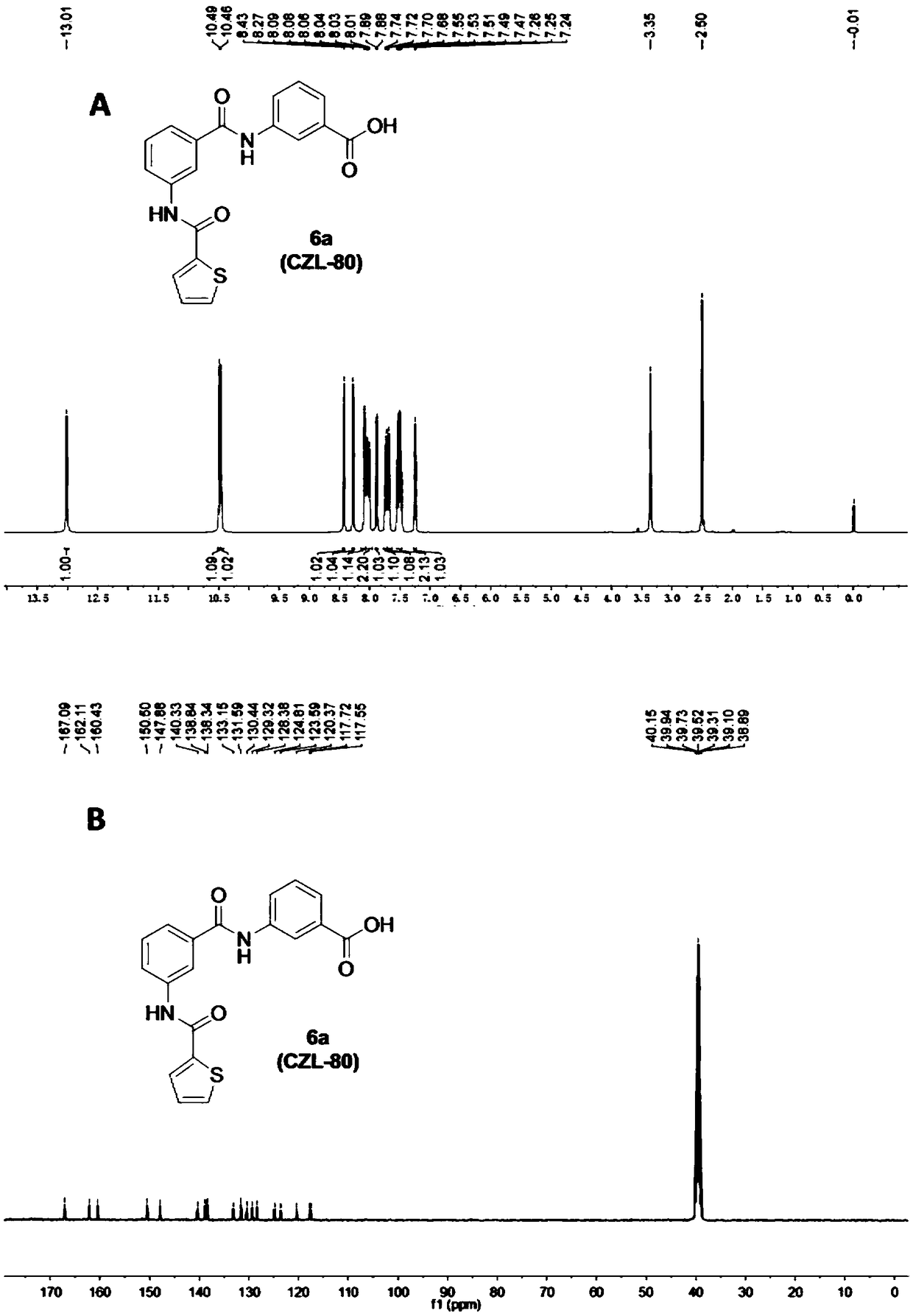
[0050] Step A: Add thiophene-2-carboxylic acid (1.024 g, 8 mmol) into a 25 mL one-necked bottle, add thionyl chloride (5 mL), and heat up to reflux for 1 hour. After the reaction, the thionyl chloride was removed under reduced pressure, the oil pump was drained, anhydrous tetrahydrofuran was added to dissolve, and 3-aminobenzoic acid (1a) (1.097g, 8mmol) (1.097g, 8mmol) and triethylamine (0.808 g, 8mmol), reacted at room temperature for 2 hours. After the reaction, THF was removed under reduced pressure, the residue was added with water and extracted three times with 10 mL of ethyl acetate, the organic phase was washed three times with 10 mL of saturated brine, the organic phase was removed under reduced pressure for ethyl acetate, and the oil pump was drained to obtain a white solid 3-( Thiophene-2-carboxamido)benzoic acid (2a), yield 95%.
[0051] Step B: Dissolve 3-aminobenzoic acid (1a...
Embodiment 2
[0054] Example 2 Ethyl 3-(3-(thiophene-2-carboxamido)benzamido)benzoate
[0055]
[0056] Synthesis steps Referring to Example 1, 5b was synthesized using 3-aminobenzoic acid, ethanol, and thiophene-2-carboxylic acid as raw materials.
[0057] Compound 5b NMR data 1 HNMR (400MHz, Acetone-d 6 )δ9.81(s,1H),9.73(s,1H),8.51(s,1H),8.36(s,1H),8.19(d,J=8.1Hz,1H),8.06(d,J=8.0 Hz,1H),7.99(d,J=3.6Hz,1H),7.79(m,3H), 7.51(m,2H),7.28–7.12(m,1H),4.37(q,J=7.1Hz,2H ),1.38(t,J=7.1Hz,3H); 13 CNMR (100MHz, Acetone-d 6 )δ166.58,166.37,160.98,141.08,140.62,140.24,136.73,132.48,132.01, 129.75,129.44,128.77,125.35,125.31,124.06,123.44,121.81,120.32,61.54,14.60;ESI-MS:m / z =395 [M+1 + ].
Embodiment 3
[0058] Example 3 3-(3-(thiophene-2-carboxamido)benzamido)isopropyl benzoate
[0059]
[0060] Synthesis steps Referring to Example 1, 5c was synthesized using 3-aminobenzoic acid, isopropanol, and thiophene-2-carboxylic acid as raw materials.
[0061] Compound 5c NMR data 1 HNMR (400MHz, Acetone-d 6 )δ9.80(s,1H),9.72(s,1H),8.48(s,1H),8.35(s,1H),8.19(d,J=8.0Hz,1H),8.06(d,J=8.1 Hz,1H),7.99(d,J=3.4Hz,1H),7.81(d,J=4.8Hz,1H),7.77(d,J=7.7Hz,2H),7.50(t,J=7.9Hz, 2H), 7.25(m, 1H), 5.35–5.07(m, 1H), 1.37(d, J = 6.2Hz, 6H); 13CNMR (100MHz, Acetone-d 6 )δ166.35,166.08,160.96,141.08,140.58,140.25, 136.75,132.48,132.39,129.75,129.70,129.43,128.77,125.34,125.26,124.03,123.42,121.81, 120.31,68.99,22.10;ESI-MS:m / z =409[M+1 + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com