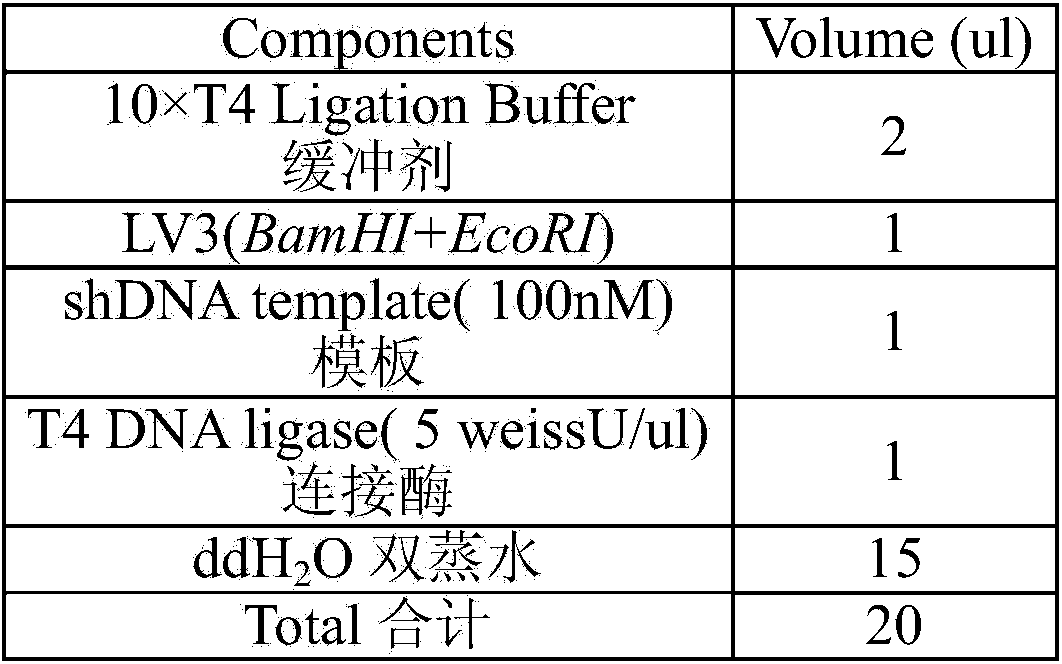
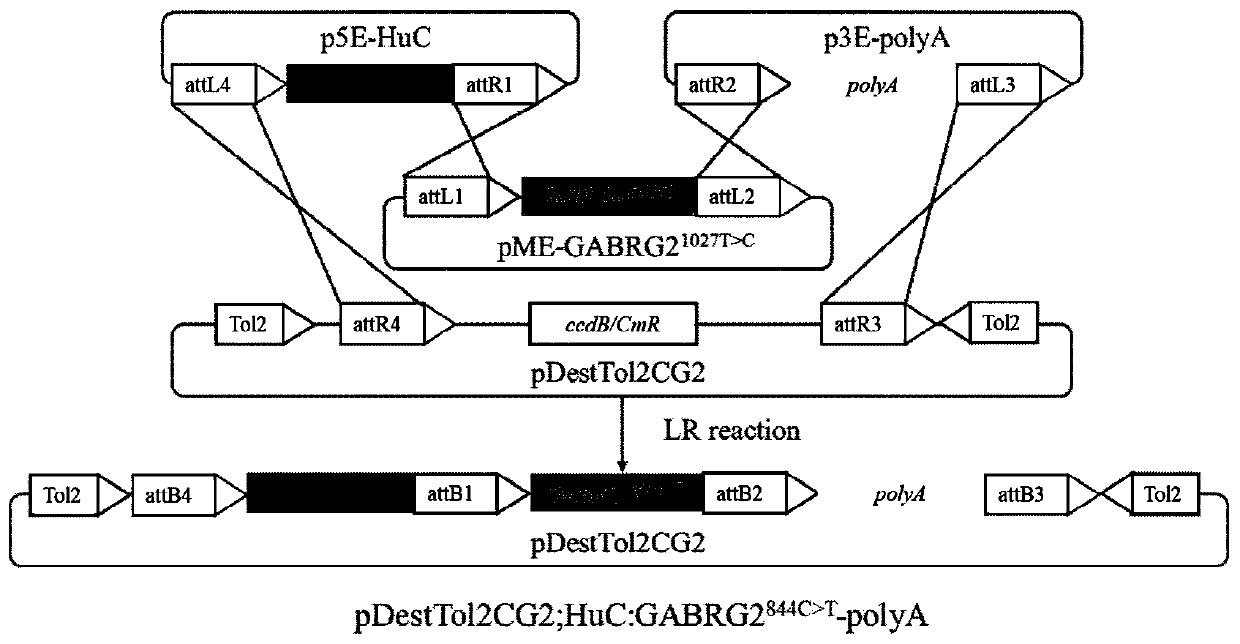
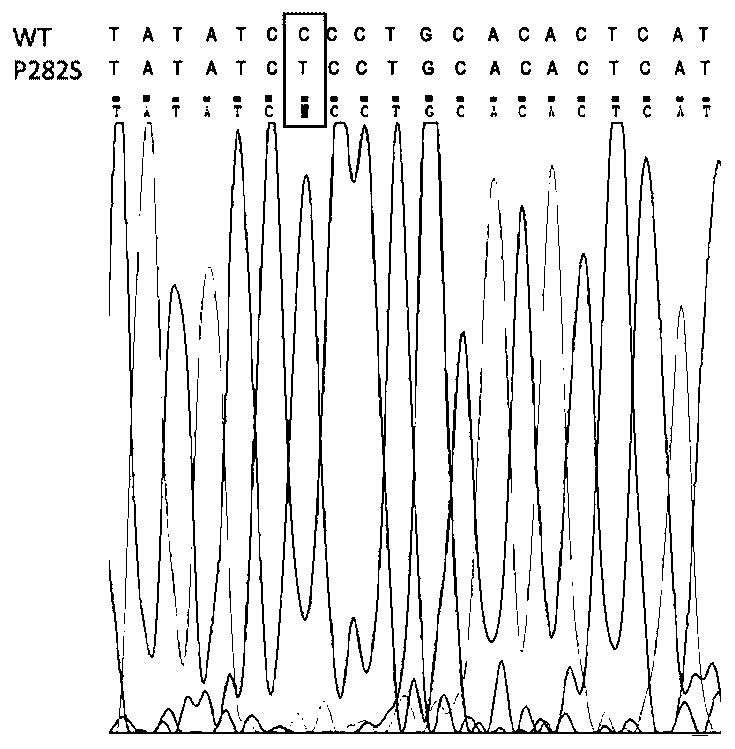
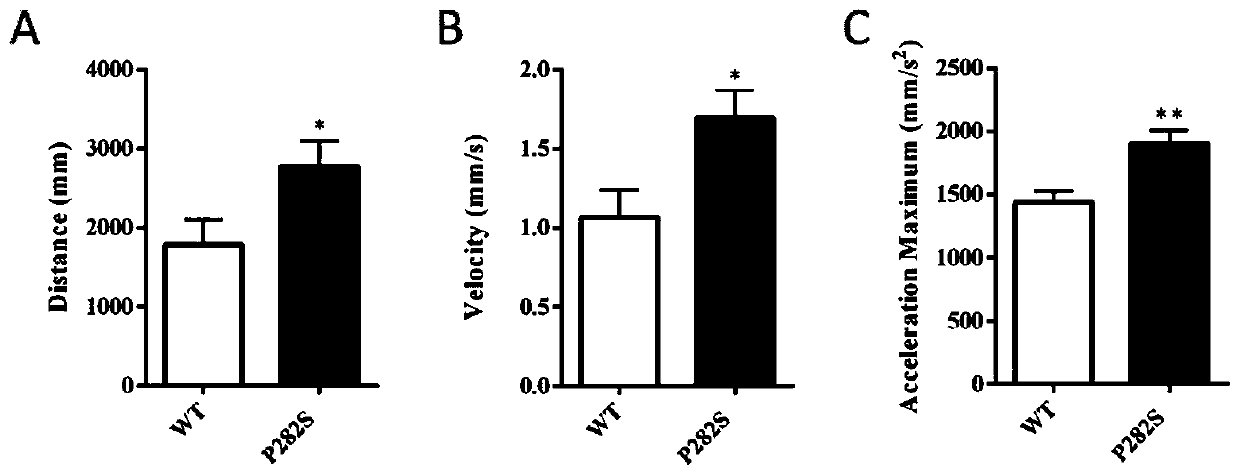
Patents
Literature
36 results about "Refractory epilepsy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
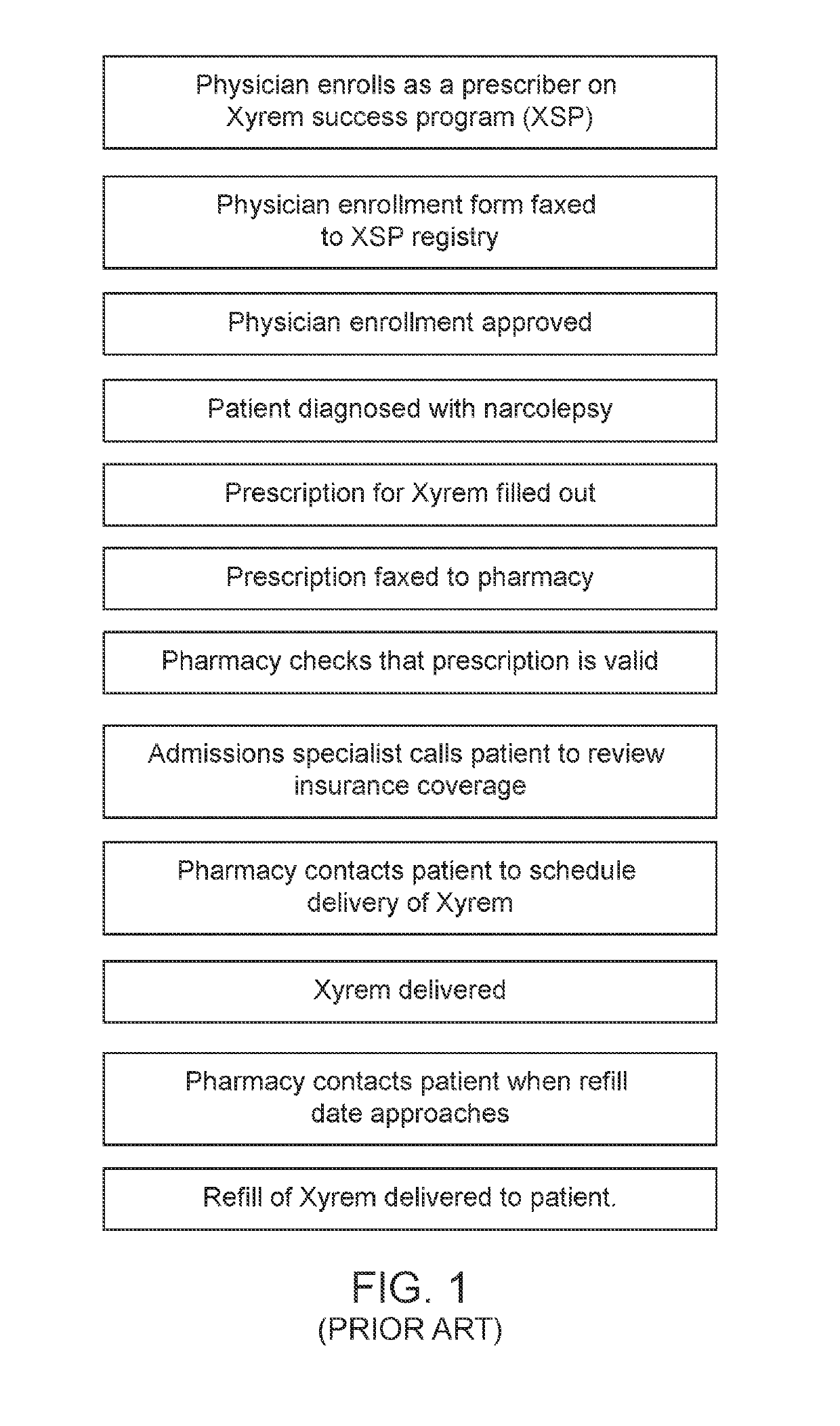
Refractory epilepsy is a seizure disorder that resists drug treatment. There is some debate among clinicians and researchers about how to define refractory epilepsy.
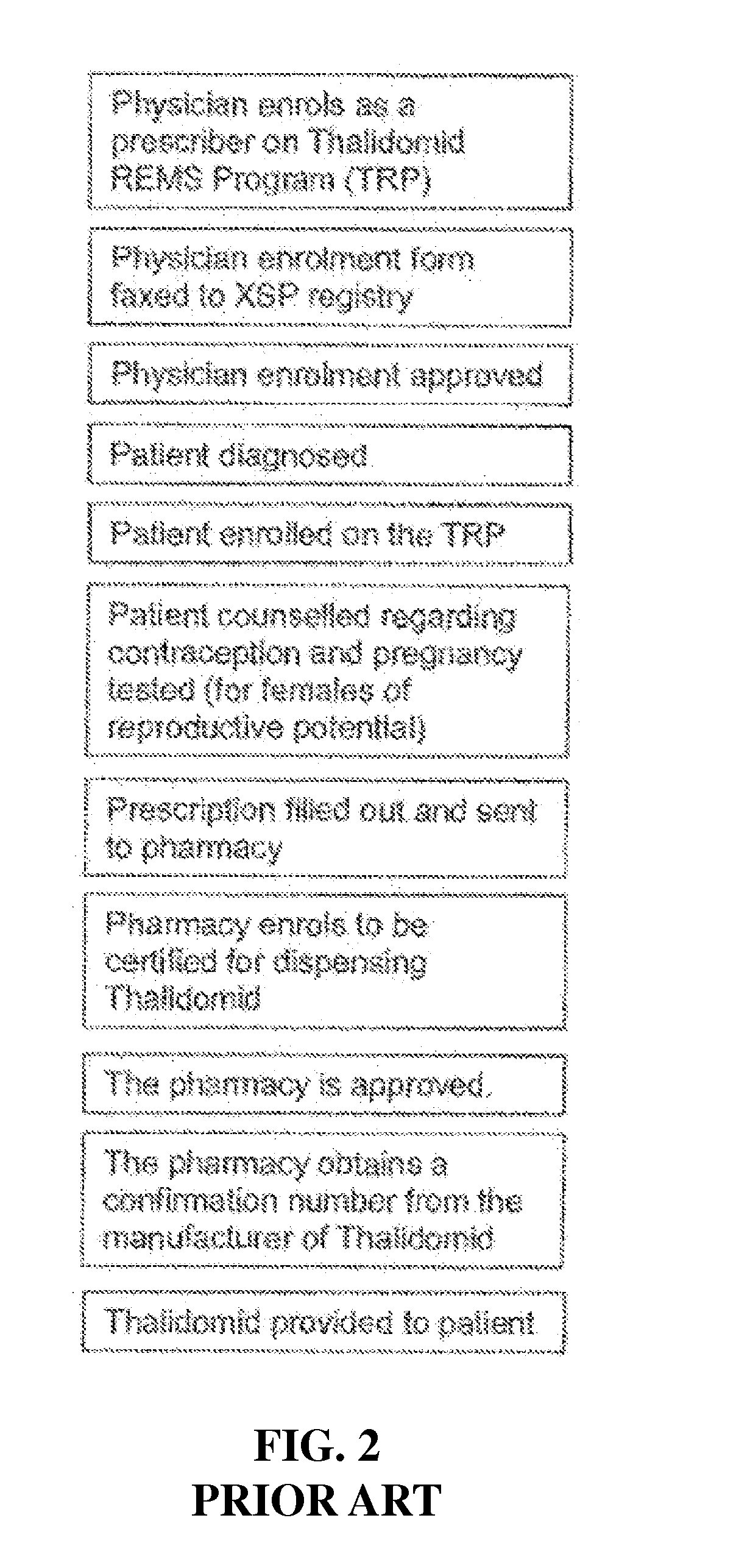
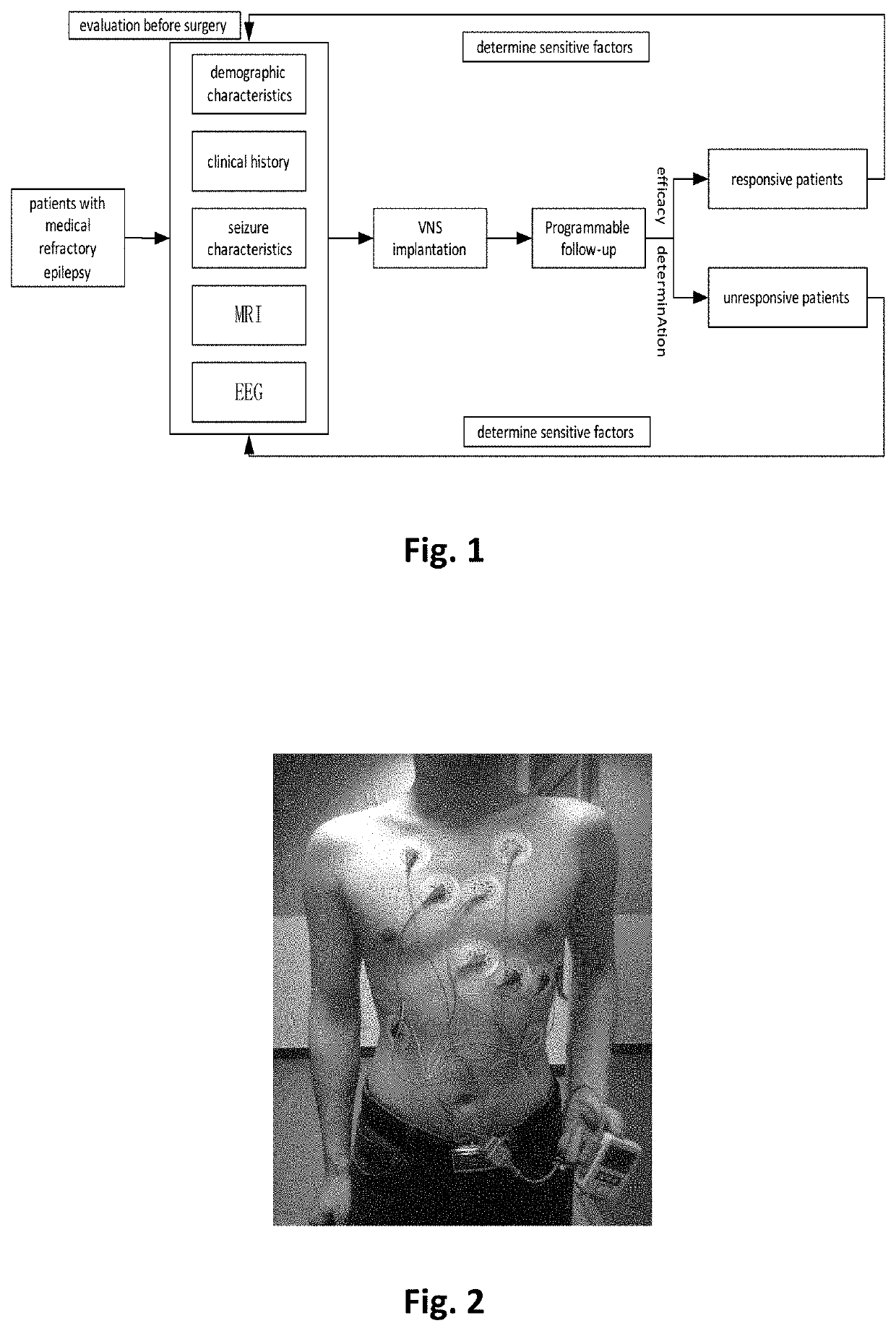
Computatonal tool for pre-surgical evaluation of patients with medically refractory epilepsy
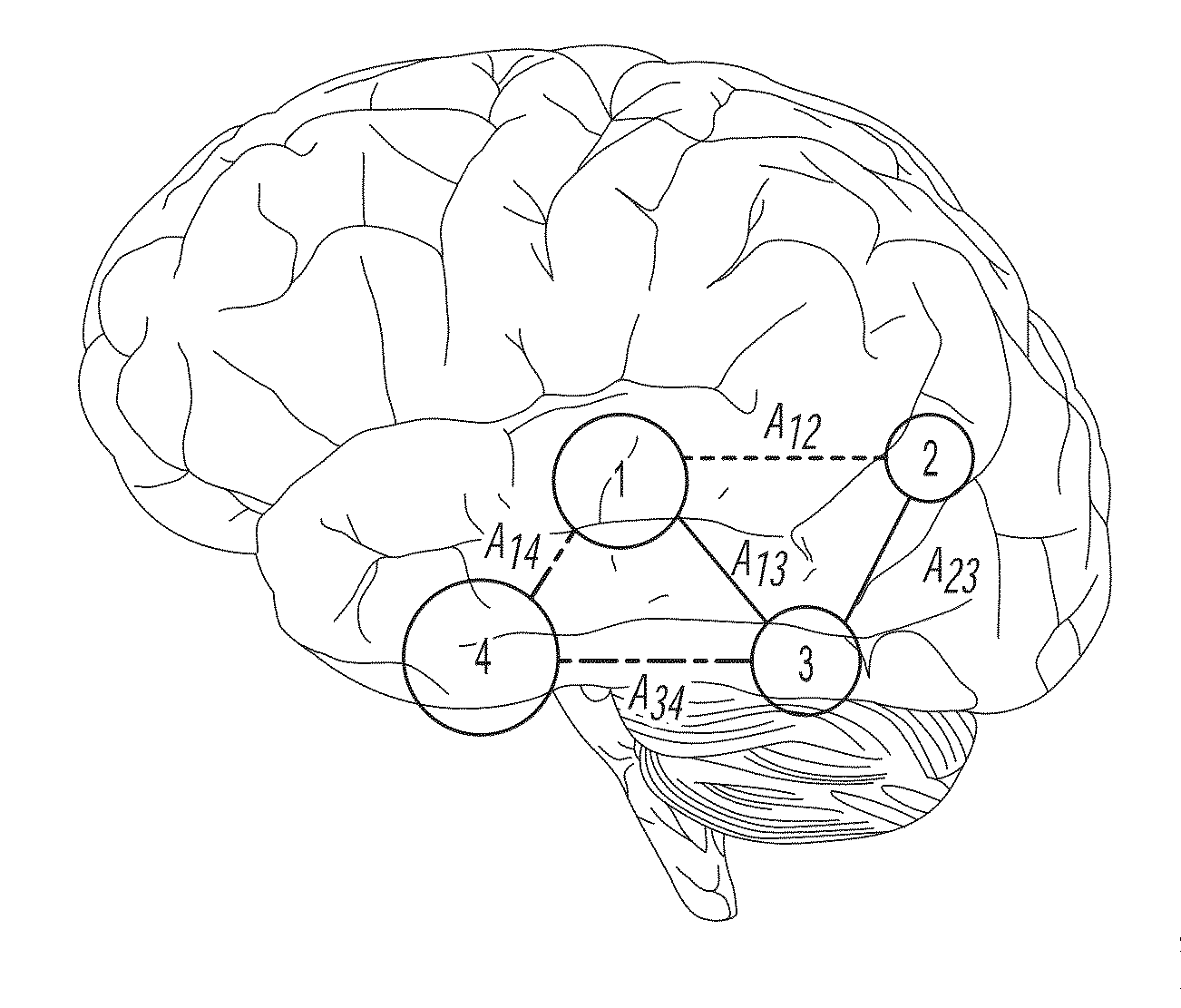
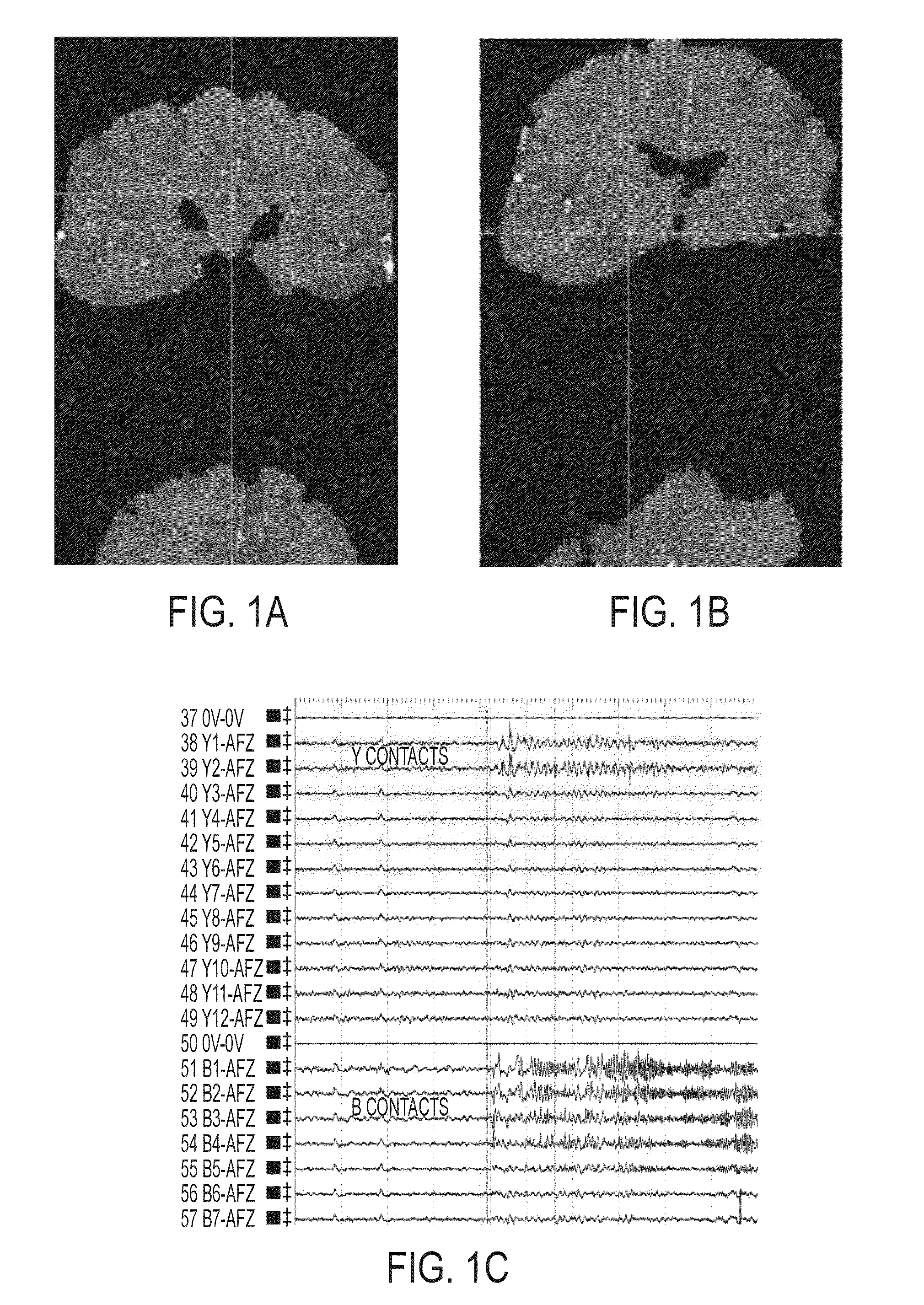
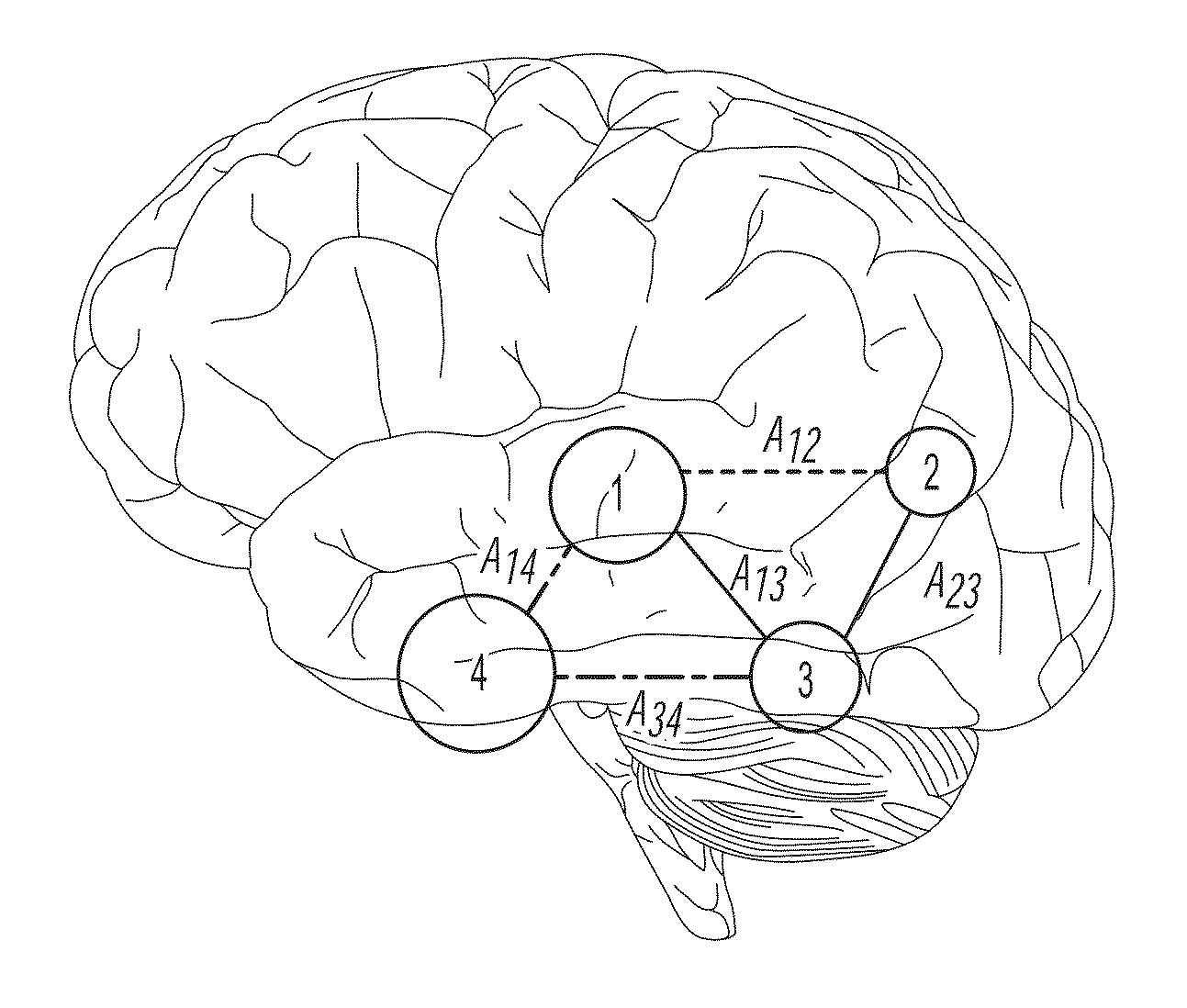
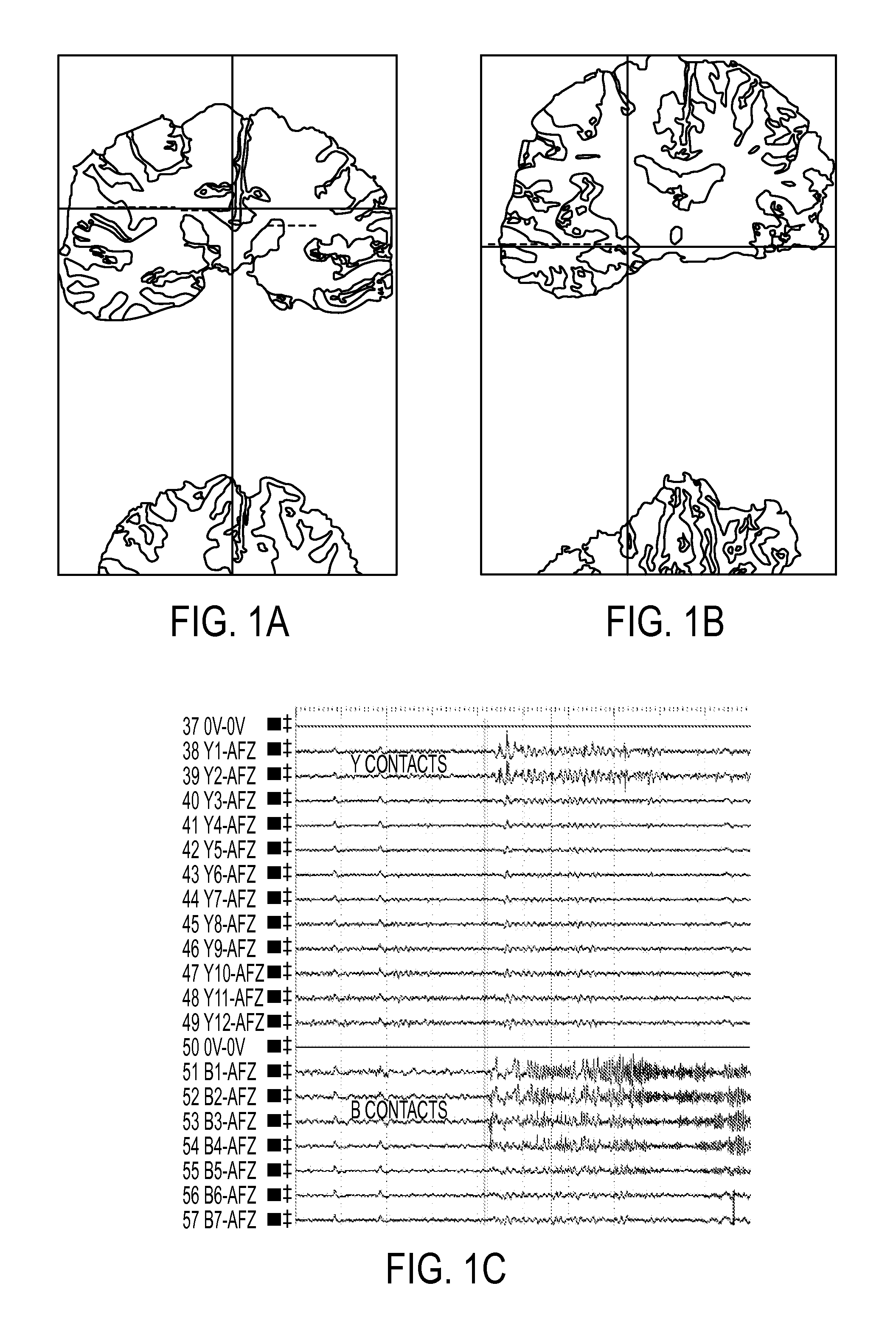
A method of identifying an epileptogenic zone of a subject's brain includes receiving a plurality of electrical signals from a corresponding plurality of surgically implanted electrodes, calculating a first plurality of connectivities between each pair of electrodes based on a portion of each of the plurality of electrical signals corresponding to a first time period, assigning a rank corresponding to each electrode for the first period of time based on the first plurality of connectivities to provide a first plurality of ranks, calculating a second plurality of connectivities between each pair of electrodes based on a portion of each of the plurality of electrical signals corresponding to a second time period, assigning a rank corresponding to each electrode for the second period of time based on the second plurality of connectivities to provide a second plurality of ranks, identifying a cluster of electrodes among the plurality of electrodes based on relative changes between the first plurality of ranks from the first time period and the second plurality of ranks at the second time period, and identifying the epileptogenic zone based on the cluster of electrodes.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Method and system for predicting refractory epilepsy status
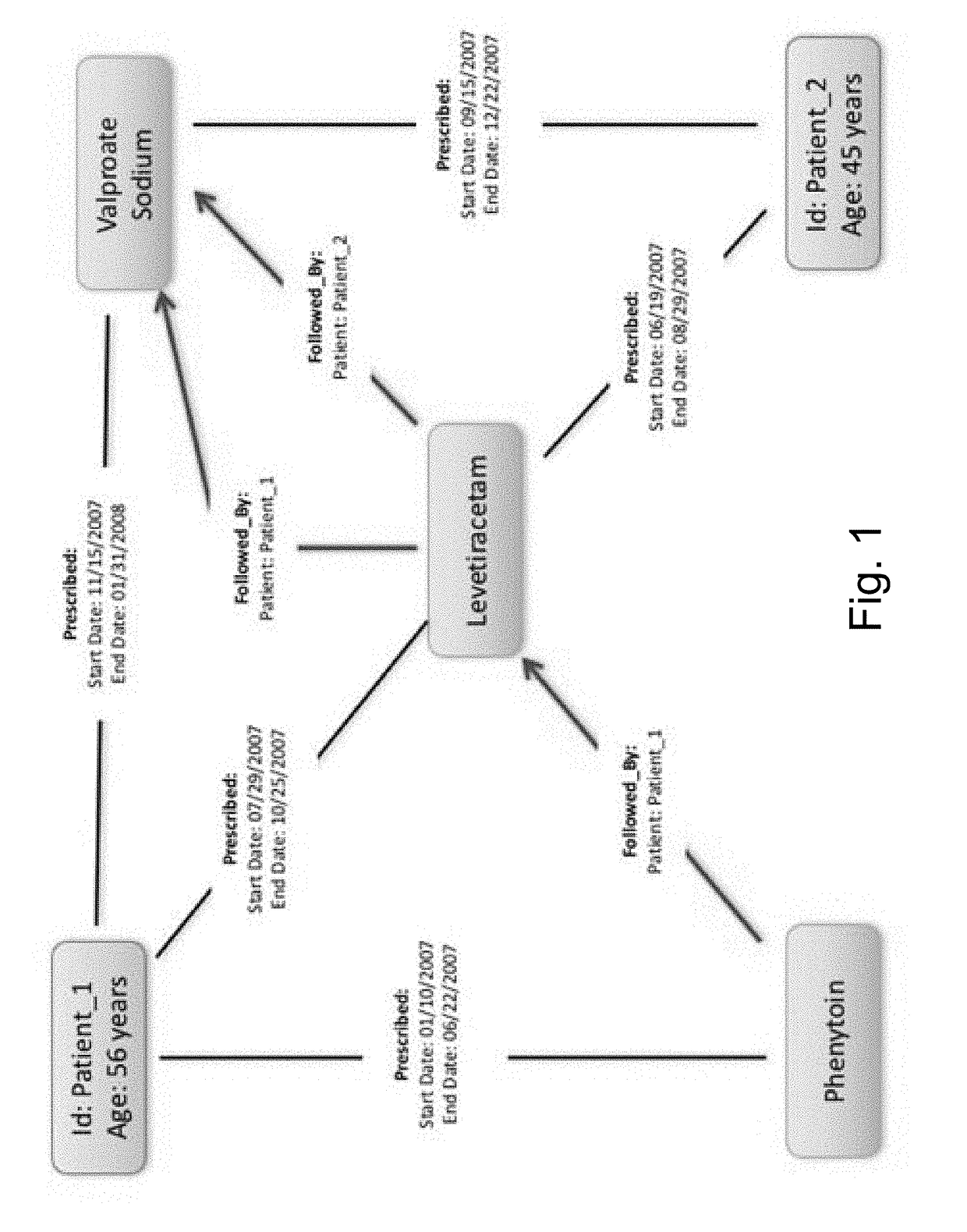
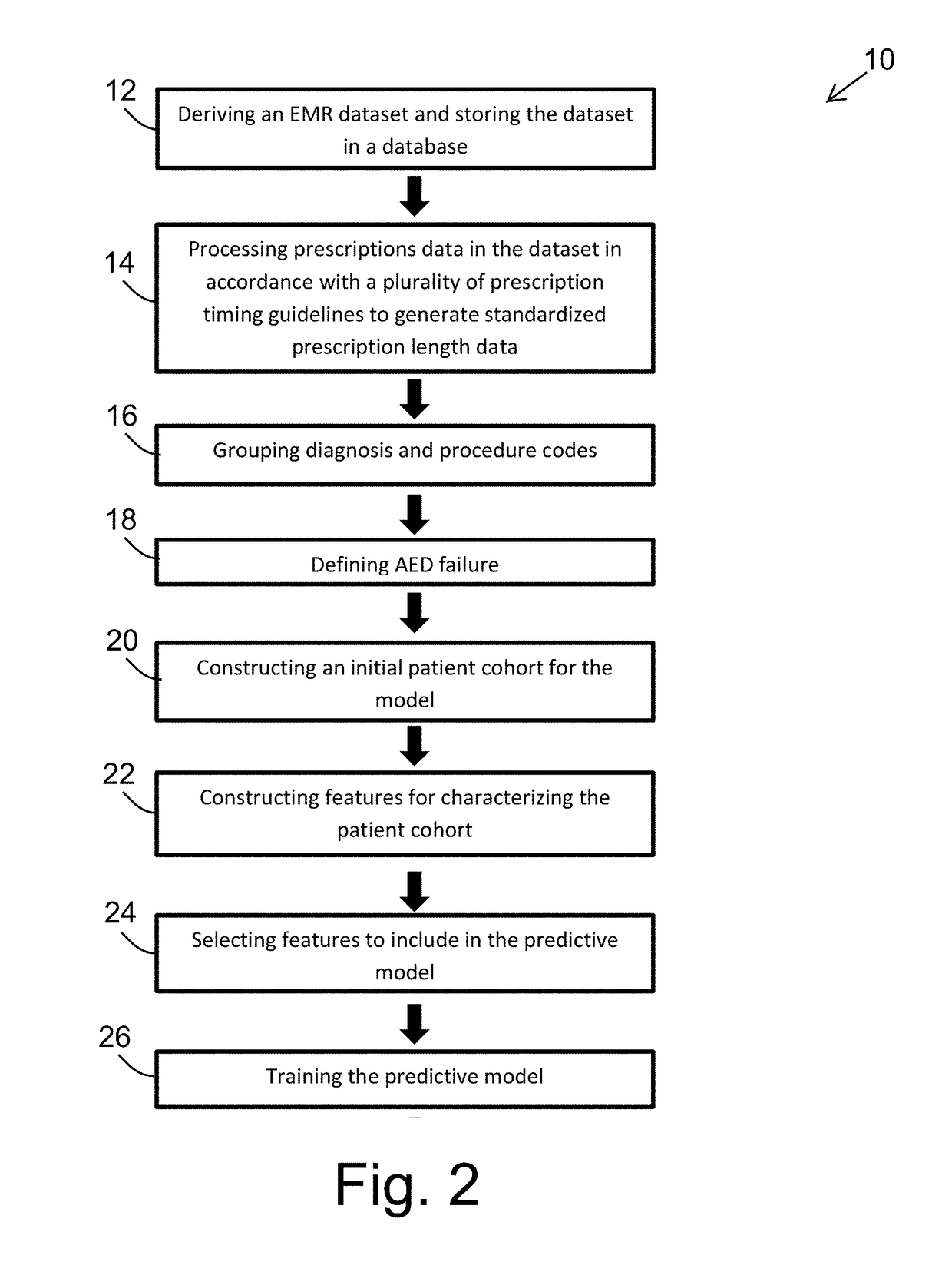
A method of building a machine learning pipeline for predicting refractoriness of epilepsy patients is provided. The method includes providing electronic health records data; constructing a patient cohort from the electronic health records data by selecting patients based on failure of at least one anti-epilepsy drug; constructing a set features found in or derived from the electronic health records data; electronically processing the patient cohort to identify a subset of the features that are predictive for refractoriness for inclusion in a predictive model configured for classifying patients as refractory or non-refractory; and training the predictive computerized model to classify the patients having at least one anti-epilepsy drug failure based on likelihood of becoming refractory.
Owner:UCB PHARMA SRL
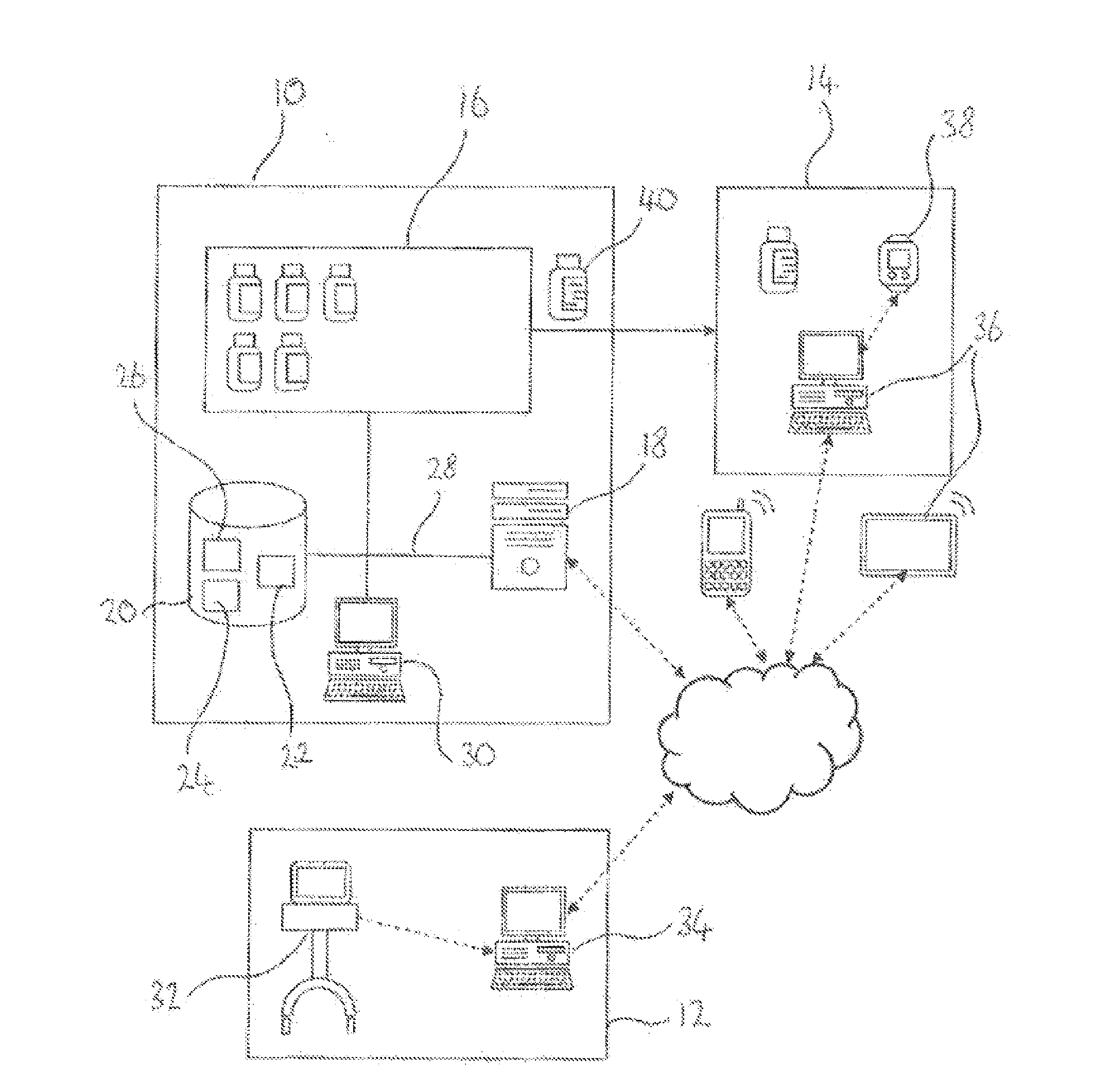
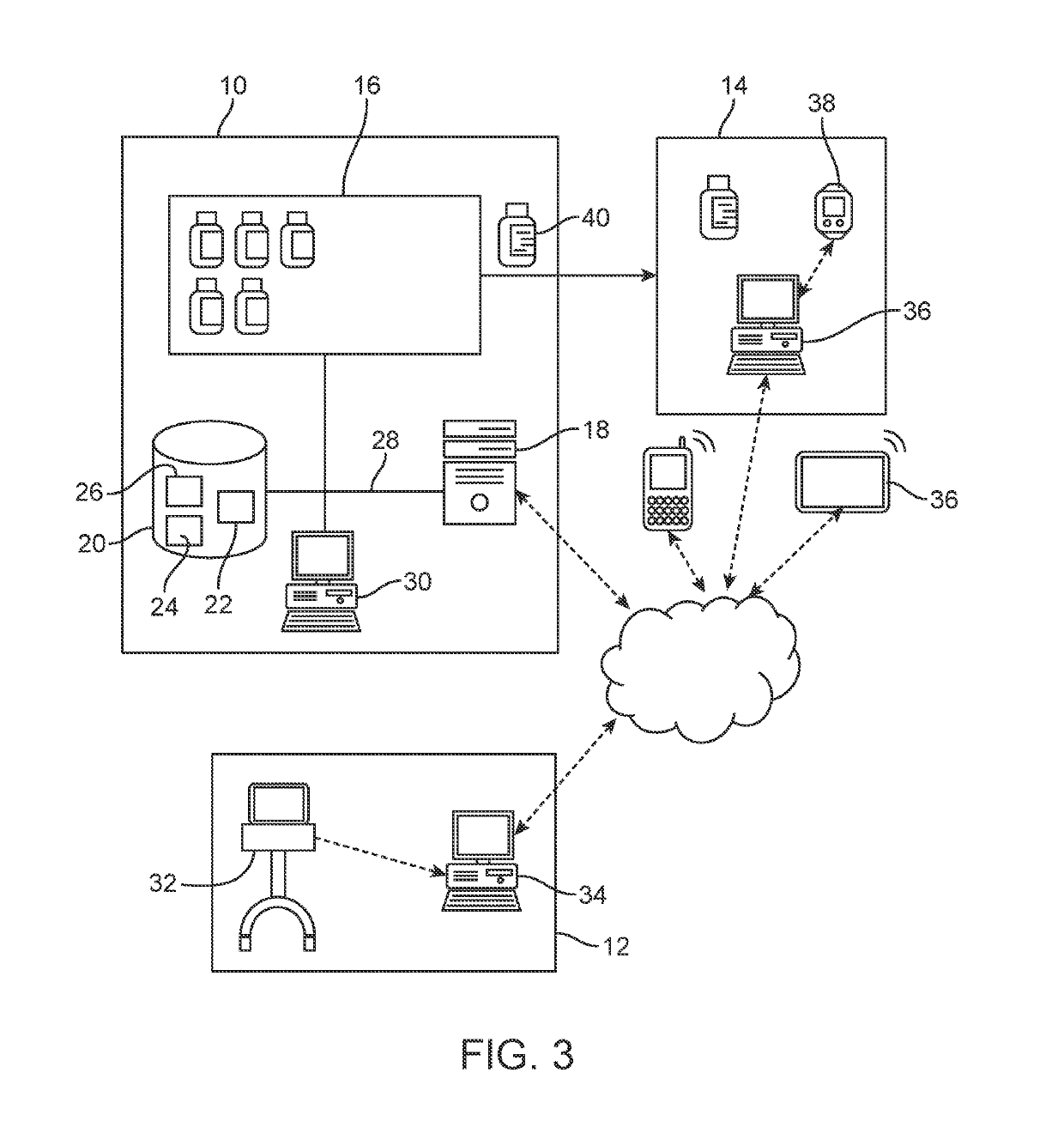
Control system for control of distribution of medication
ActiveUS20160092652A1Improve understandingReduce and prevent instance of misdiagnosisData processing applicationsDigital data information retrievalMedication authorizationMedicine
A system of controlling distribution of a medication in the treatment or prevention of epilepsy is provided. A central controller of the system has a data store and one or more processors for reading and writing data to the data store. The data store comprises a database of patient records, each patient record having a medication authorization field. The central controller can output an authorization of a first prescription of epilepsy medication to a patient in dependence upon genetic test results for the patient and schedules a subsequent test for the patient prior to authorization of a subsequent prescription of epilepsy medication. Also provided are methods in which the subject systems find use. The systems and methods find use in the treatment of severe subtypes of epilepsy or refractory epilepsy, such as Dravet Syndrome.
Owner:ZOGENIX INT
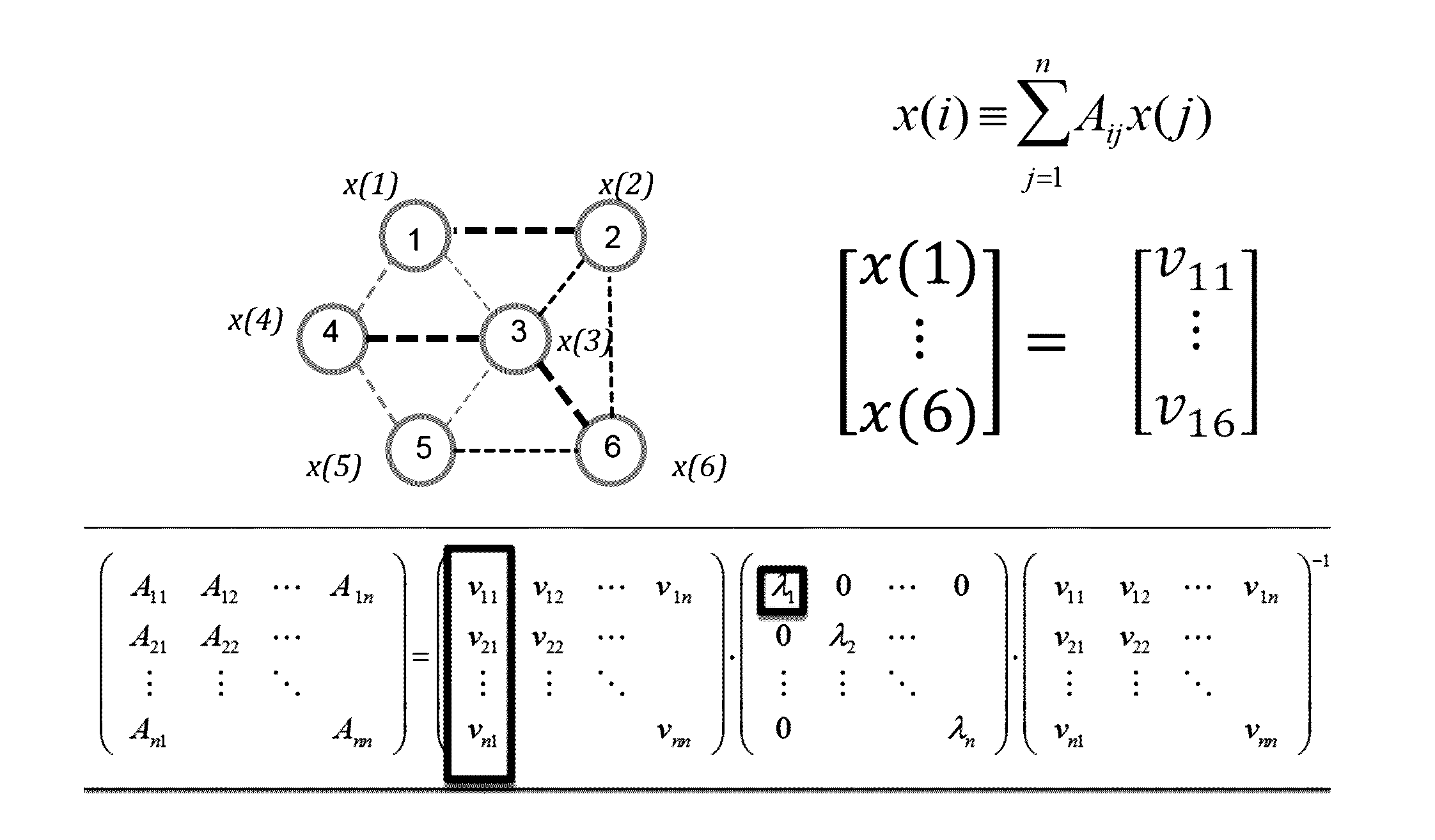
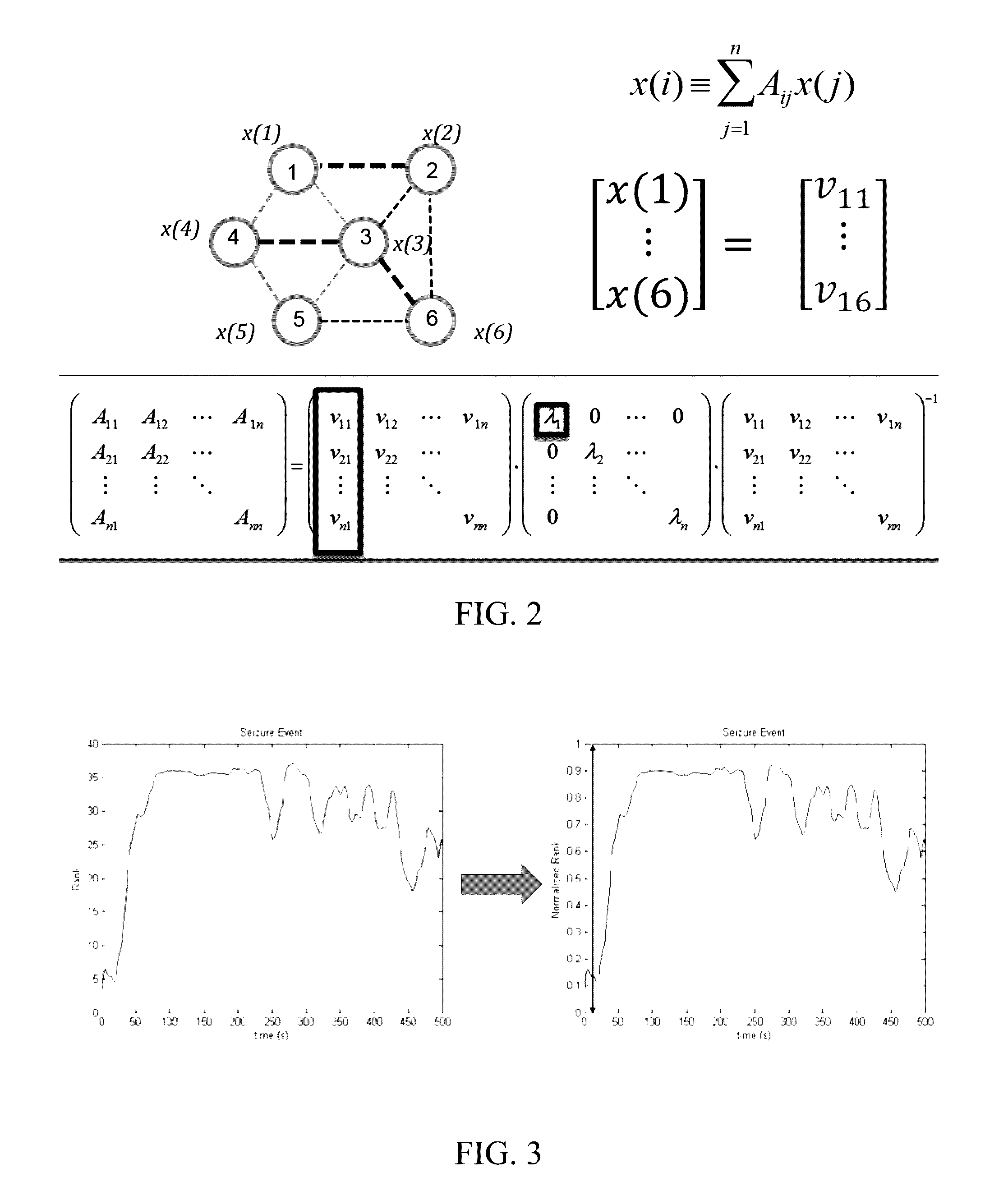
Computational tool for pre-surgical evaluation of patients with medically refractory epilepsy
A method of identifying an epileptogenic zone of a brain includes receiving a plurality of electrical signals from a plurality of surgically implanted electrodes, calculating components of an adjacency matrix, calculating eigenvectors from the adjacency matrix, and selecting an eigenvector having a largest eigenvalue. The method includes assigning an integer rank to each component of the eigenvector, sliding the time window by a time increment and repeating the immediately preceding steps a plurality of times. The method includes normalizing each rank signal, extracting a multidimensional feature vector from each normalized signal, projecting each multidimensional feature vector onto a reduced dimensionality space, and receiving a plurality of training data points represented in the reduced dimensionality space. The method includes calculating grid weights for each feature vector, and assigning a numerical value to each electrode as an indication of whether the electrode is in an epileptogenic zone of the brain.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE +1
Control system for control of distribution of medication
ActiveUS10452815B2Reduce and prevent instance of misdiagnosisSignificant health implications for the patientDigital data information retrievalDrug and medicationsMedication authorizationMedicine
A system of controlling distribution of a medication in the treatment or prevention of epilepsy is provided. A central controller of the system has a data store and one or more processors for reading and writing data to the data store. The data store comprises a database of patient records, each patient record having a medication authorization field. The central controller can output an authorization of a first prescription of epilepsy medication to a patient in dependence upon genetic test results for the patient and schedules a subsequent test for the patient prior to authorization of a subsequent prescription of epilepsy medication. Also provided are methods in which the subject systems find use. The systems and methods find use in the treatment of severe subtypes of epilepsy or refractory epilepsy, such as Dravet Syndrome.
Owner:ZOGENIX INT
Computatonal tool for pre-surgical evaluation of patients with medically refractory epilepsy
A method of identifying an epileptogenic zone of a subject's brain includes receiving a plurality of electrical signals from a corresponding plurality of surgically implanted electrodes, calculating a first plurality of connectivities between each pair of electrodes based on a portion of each of the plurality of electrical signals corresponding to a first time period, assigning a rank corresponding to each electrode for the first period of time based on the first plurality of connectivities to provide a first plurality of ranks, calculating a second plurality of connectivities between each pair of electrodes based on a portion of each of the plurality of electrical signals corresponding to a second time period, assigning a rank corresponding to each electrode for the second period of time based on the second plurality of connectivities to provide a second plurality of ranks, identifying a cluster of electrodes among the plurality of electrodes based on relative changes between the first plurality of ranks from the first time period and the second plurality of ranks at the second time period, and identifying the epileptogenic zone based on the cluster of electrodes.
Owner:THE JOHN HOPKINS UNIV SCHOOL OF MEDICINE
Ketogenic mix preparation suitable for treating intractable epilepsy
InactiveCN101269210APromote brain developmentPromote fat metabolismNervous disorderPeptide/protein ingredientsAdditive ingredientMedicine
The invention relate to a ketogenesis mixture preparation applicable for treating refractory epilepsy, which is characterized in that: (1) fat, protein and carbohydrate form the basic prescription, a small amount of essential vitamins, minerals and aminophenol promoting the growth and the development of the human body and brain and promoting the metabolism are added in the basic prescription; (2) in the basic preparation, the weight percentages of the components are respectively that: fat 70-80 percent, protein 15-20 percent, carbohydrate 5-10 percent; (3) the physical states of all the components in the basic preparation are powder, ointment, aqua, or solid state agent. The physical states of the ketogenesis mixture preparation are powder granule, electuary, liquid agent, tablet, capsule or other solid state agent. The ketogenesis mixture preparation aims at the special nutrition requirement of Chinese, nutritional ingredient promoting the development of the brain and the metabolism is added in, the curative efficiency on refractory epilepsy reaches 80 percent, which reaches or exceeds the curative effect of the equivalent product abroad.
Owner:邓荣光
Novel lamotrigine pharmaceutical co-crystal and preparation method thereof
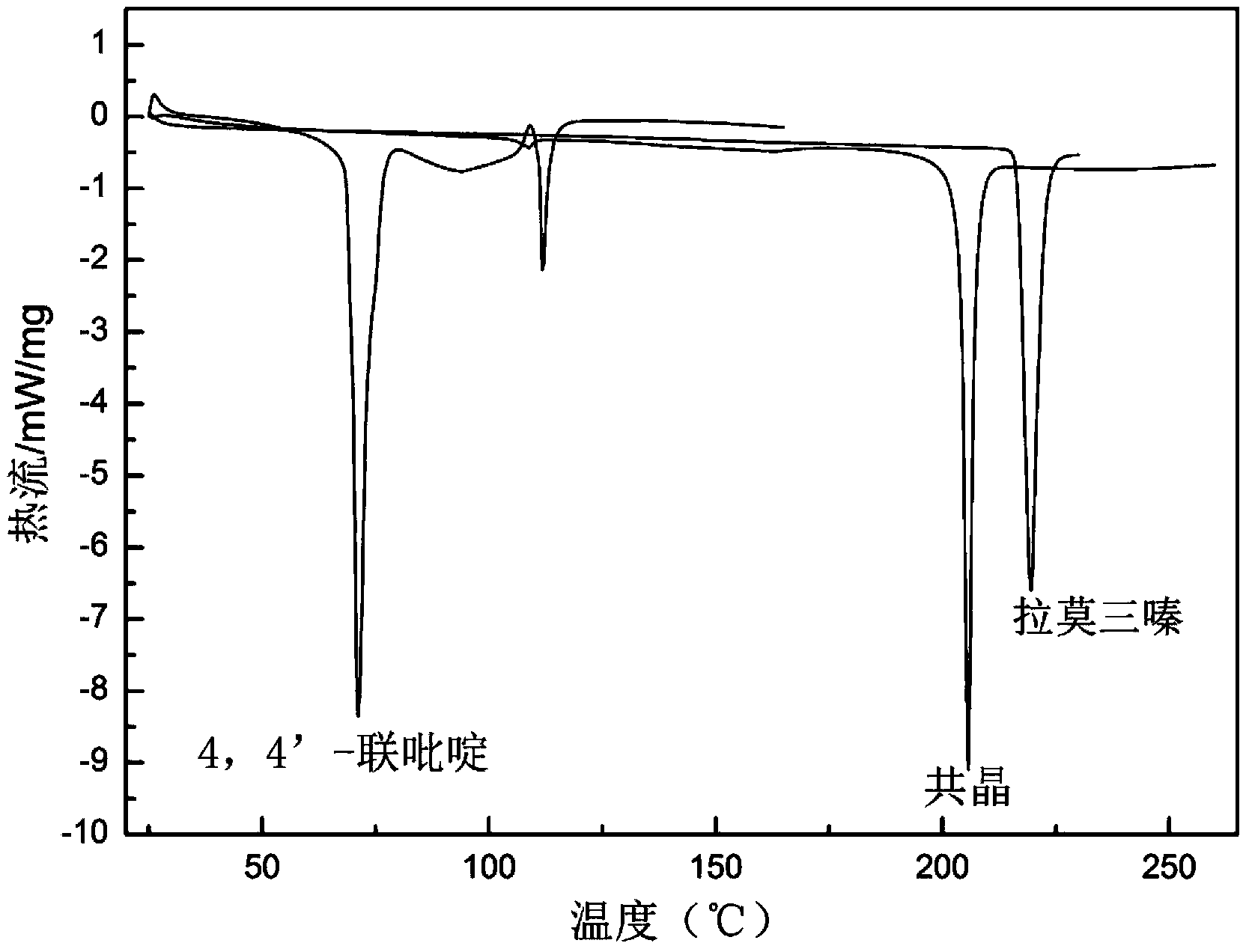
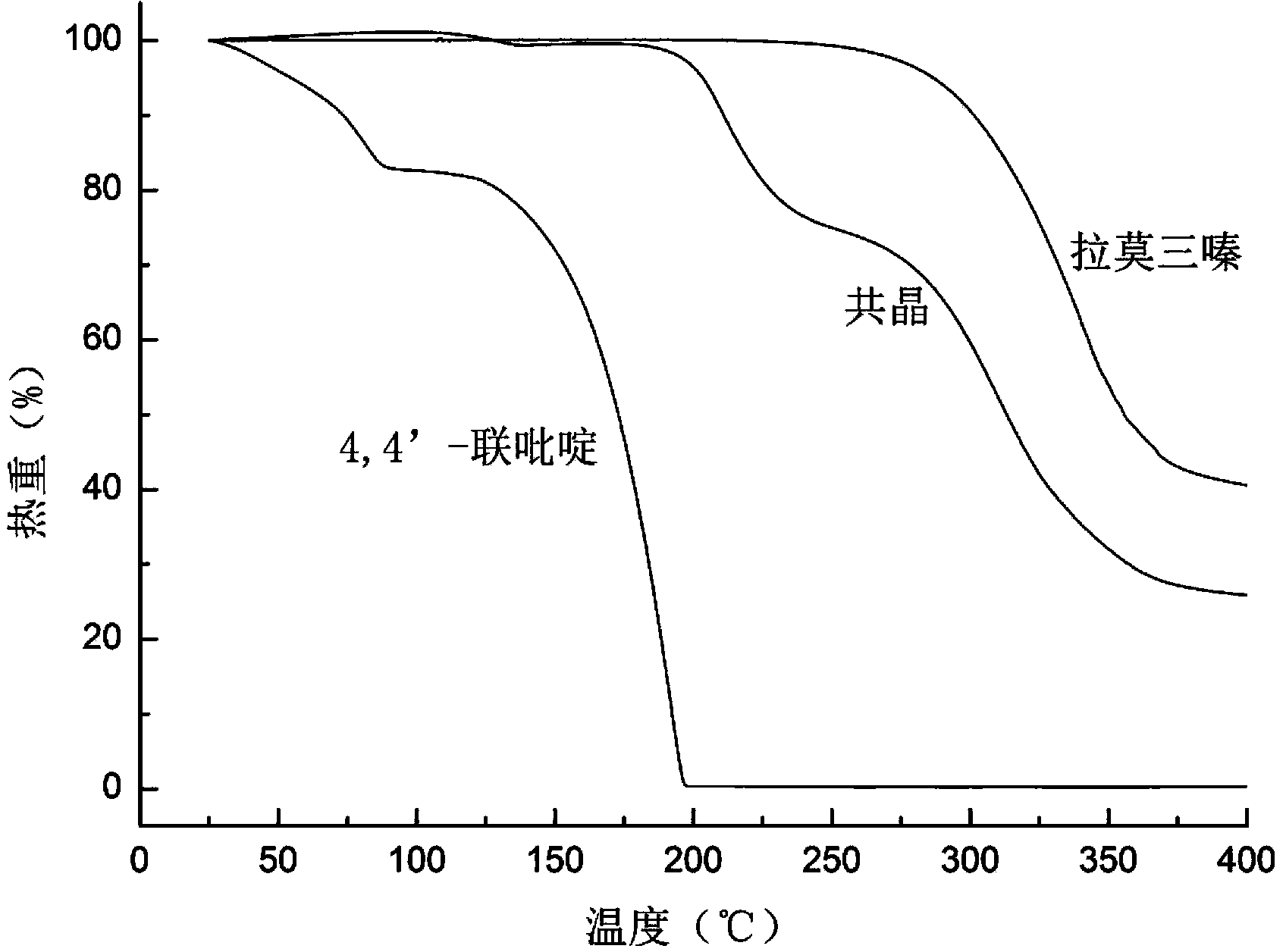
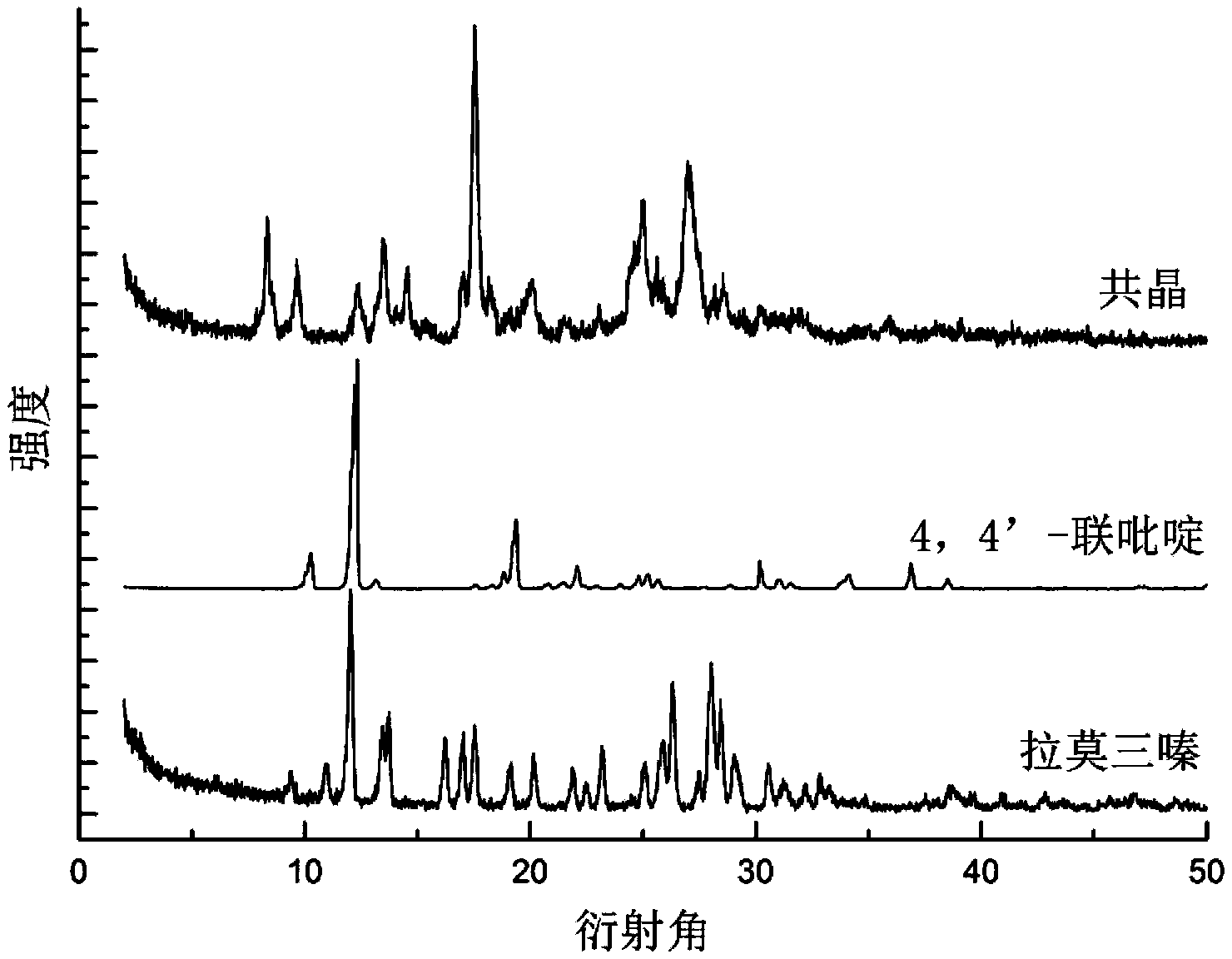
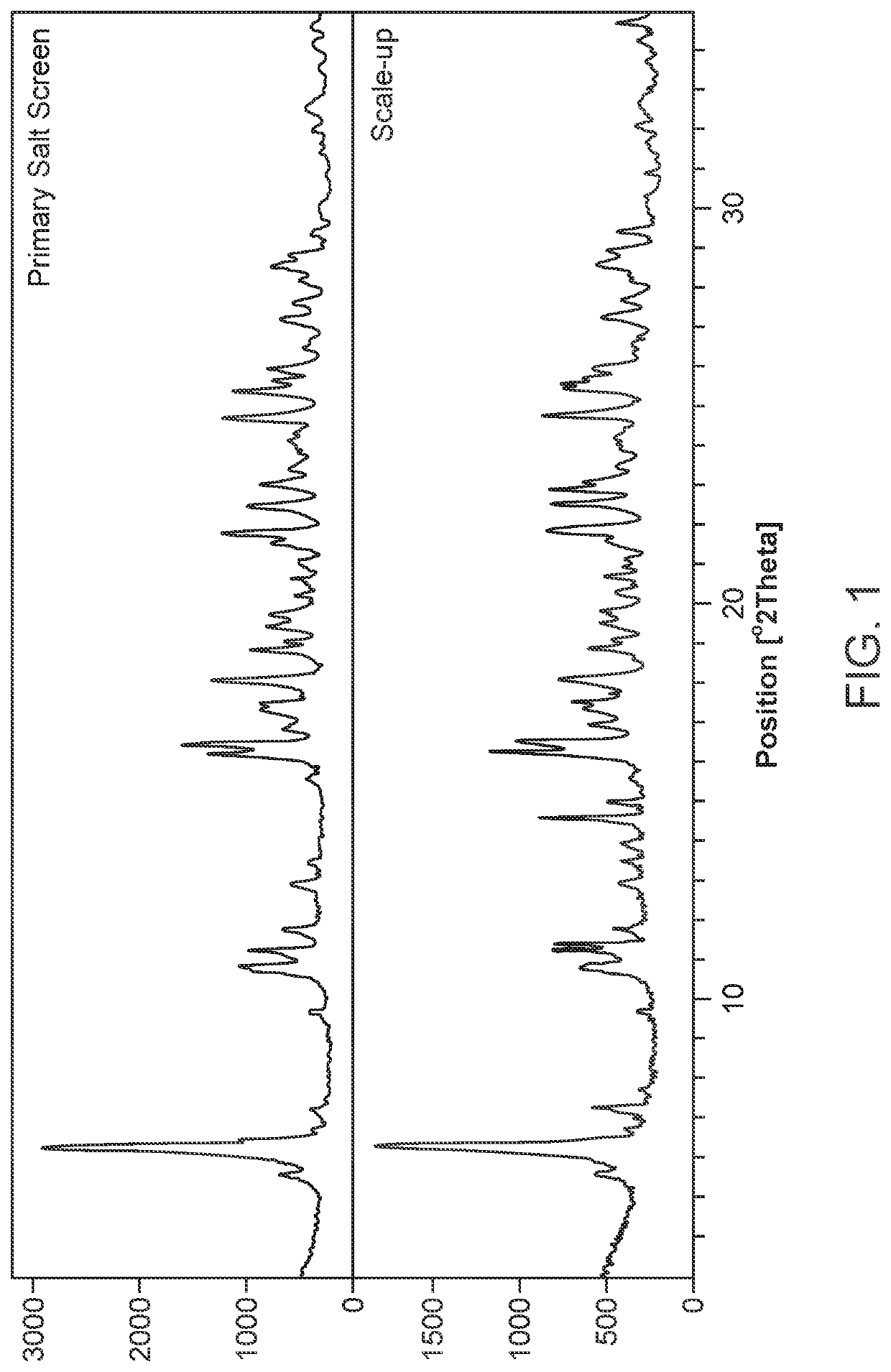
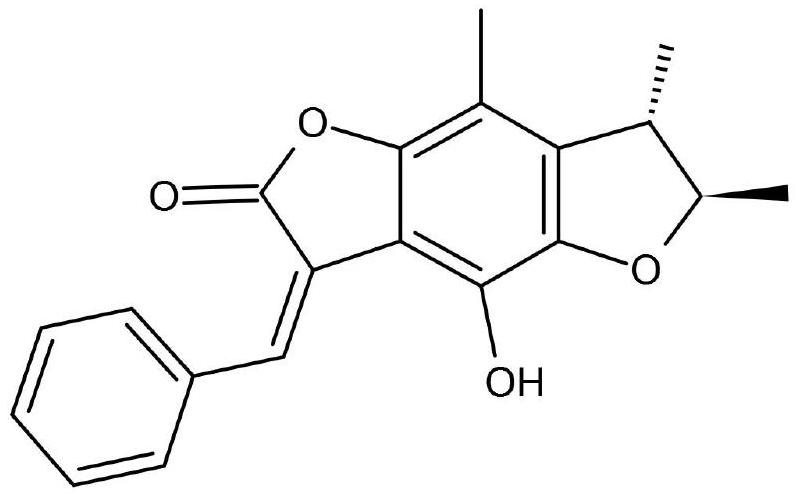
The invention relates to a novel lamotrigine pharmaceutical co-crystal and a preparation method thereof. A PXRD (Powder X Ray Diffractio) of the lamotrigine pharmaceutical co-crystal shows a series of characteristic peaks in 8.3+ / -0.2, 9.7+ / -0.2, 12.4+ / -0.2, 13.5+ / -0.2, 14.0+ / -0.2, 14.5+ / -0.2, 17.0+ / -0.2, 17.5+ / -0.2, 18.2+ / -0.2, 20.0+ / -0.2, 23.0+ / -0.2, 24.6+ / -0.2, 25.0+ / -0.2, 25.6+ / -0.2, 27.0+ / -0.2, and 28.5+ / -0.2. The lamotrigine pharmaceutical co-crystal is prepared through a solution mediate transformation or a grinding method. The prepared pharmaceutical co-crystal remains the characteristic of the traditional raw medicine on treating refractory epilepsy and also shows remarkable improvement on solubility, stability and bioavailability.
Owner:TIANJIN UNIV
Cannabinoid-containing oral pharmaceutical composition, method for preparing and using same
The present invention describes an oral pharmaceutical composition containing cannabinoid(s), an oily liquid solvent and a co-solvent, and a method for preparing and using same for the treatment of neurological disorders, especially refractory epilepsy. The analytical methods used to ensure the authenticity, quality and purity of the active pharmaceutical input and of the formulation produced werevalidated and have defined specifications.
Owner:PRATI DONADUZZI & CIA +1
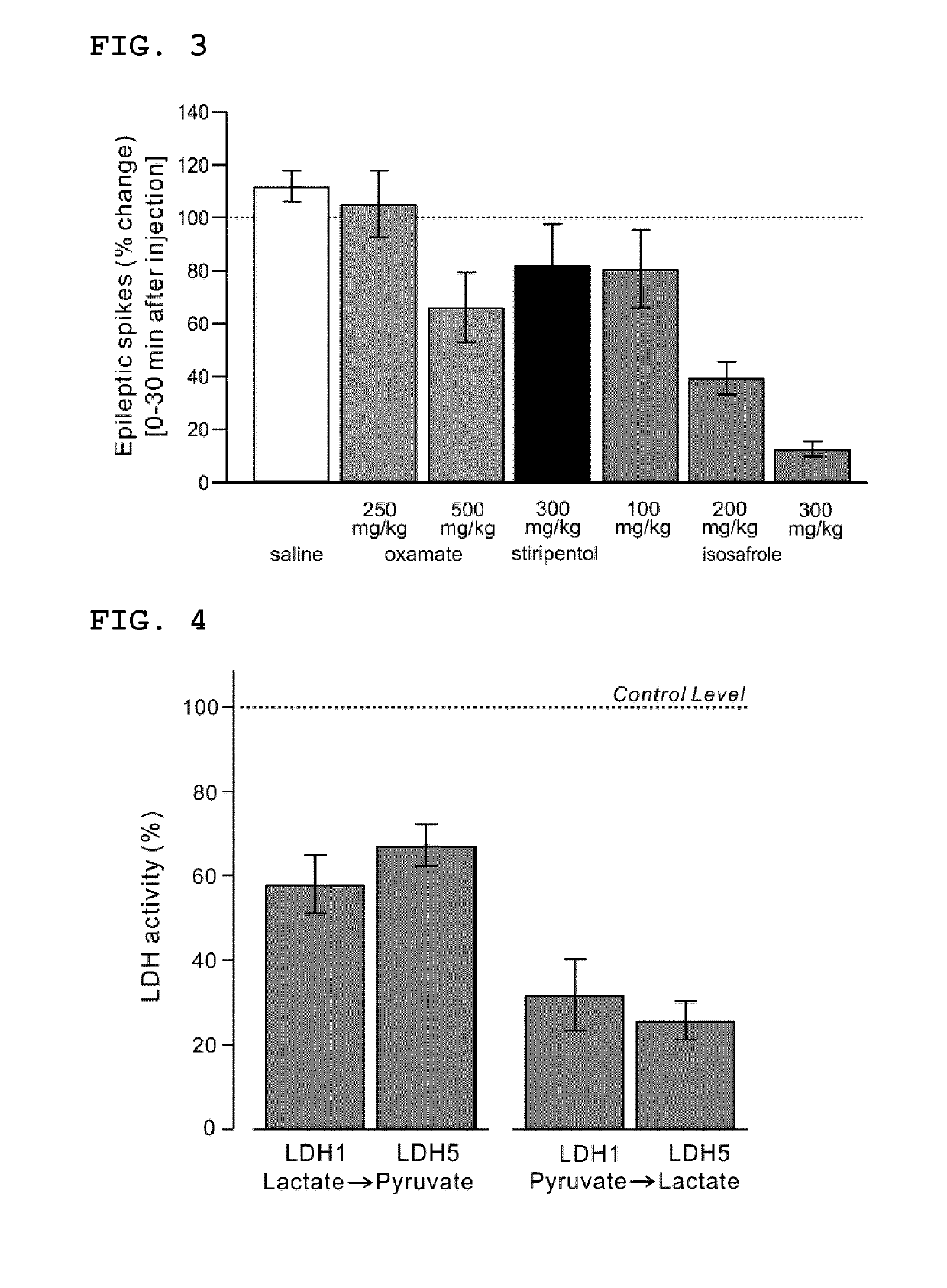
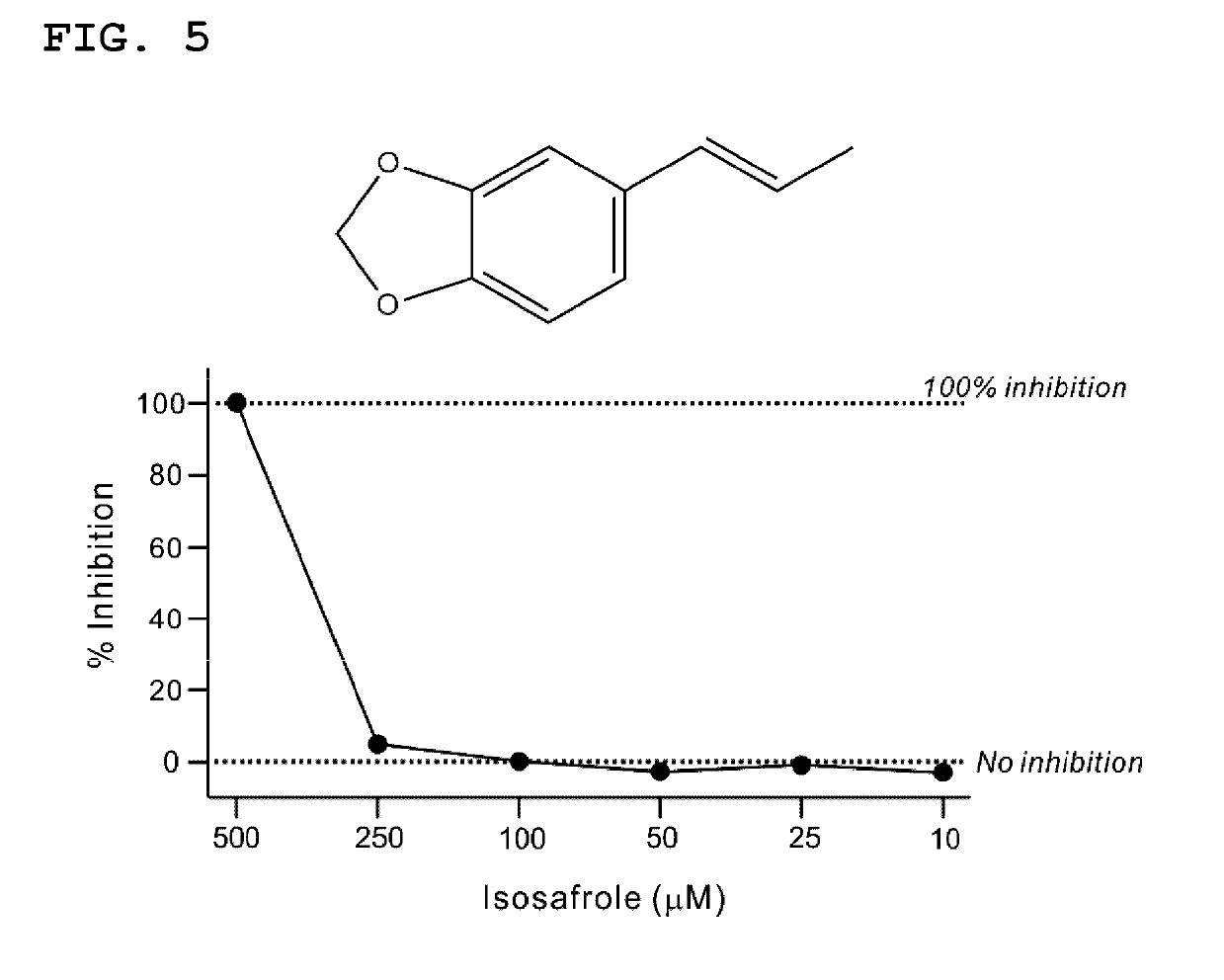
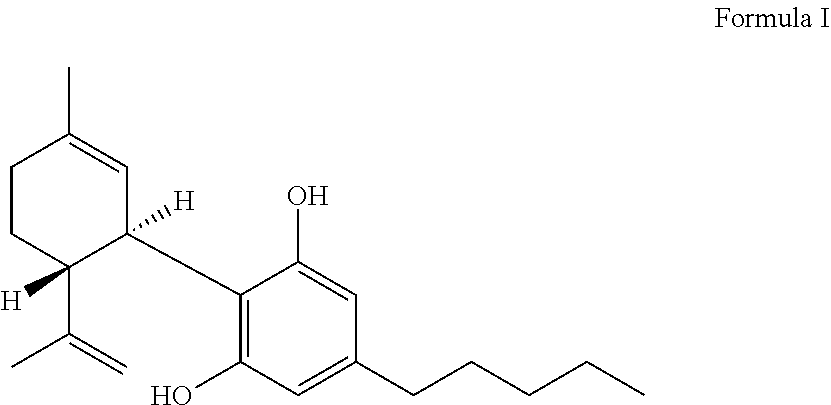
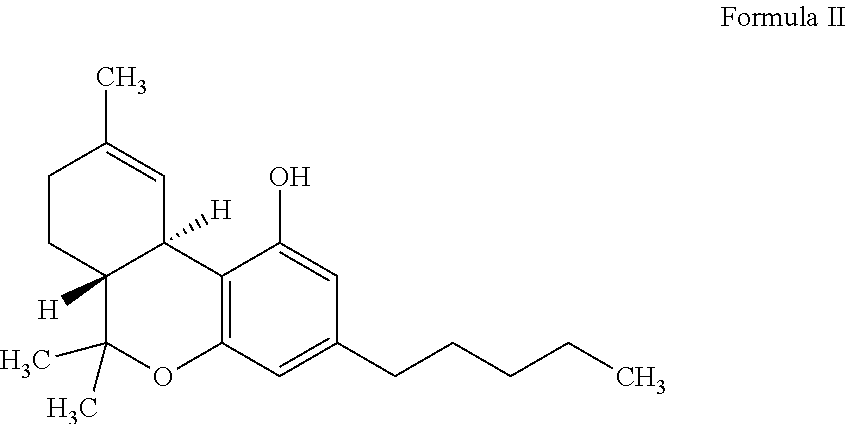
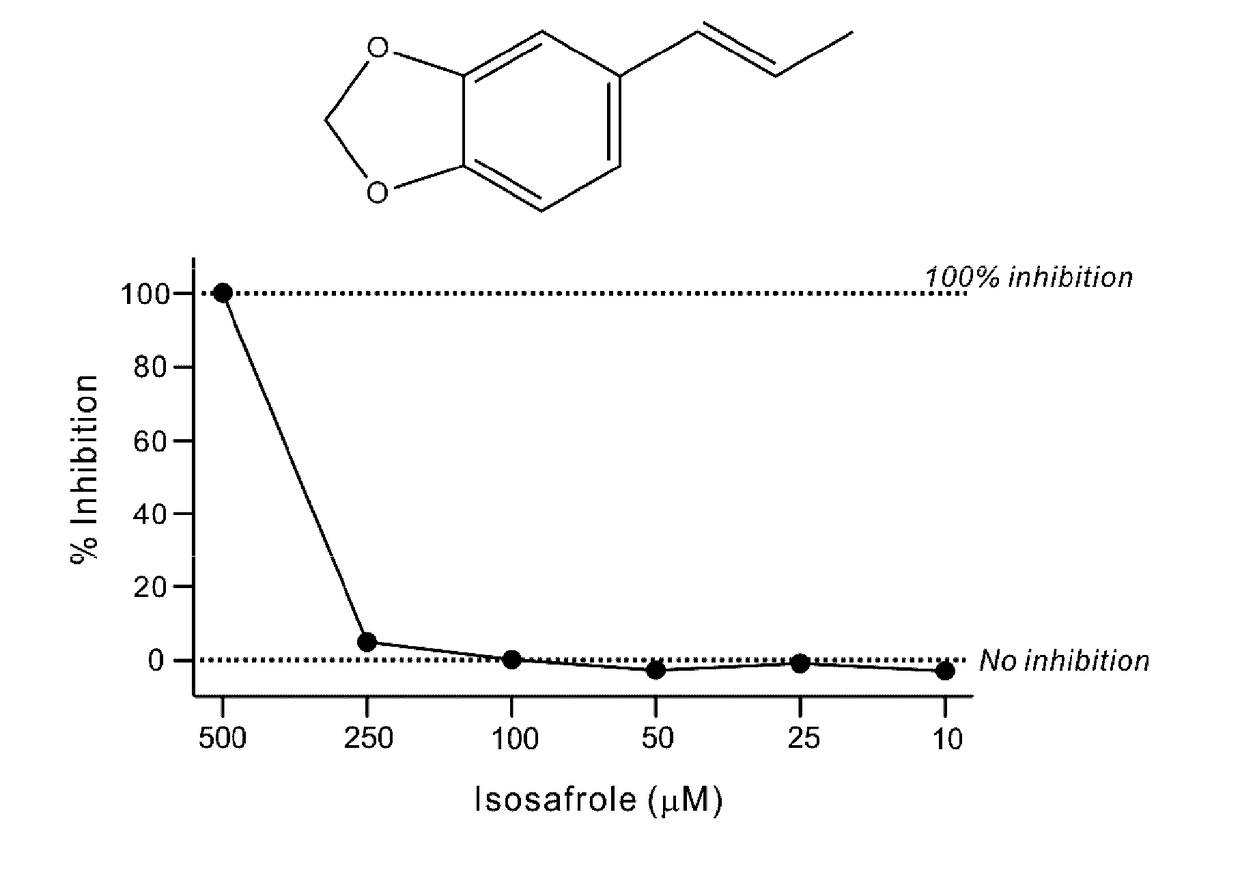
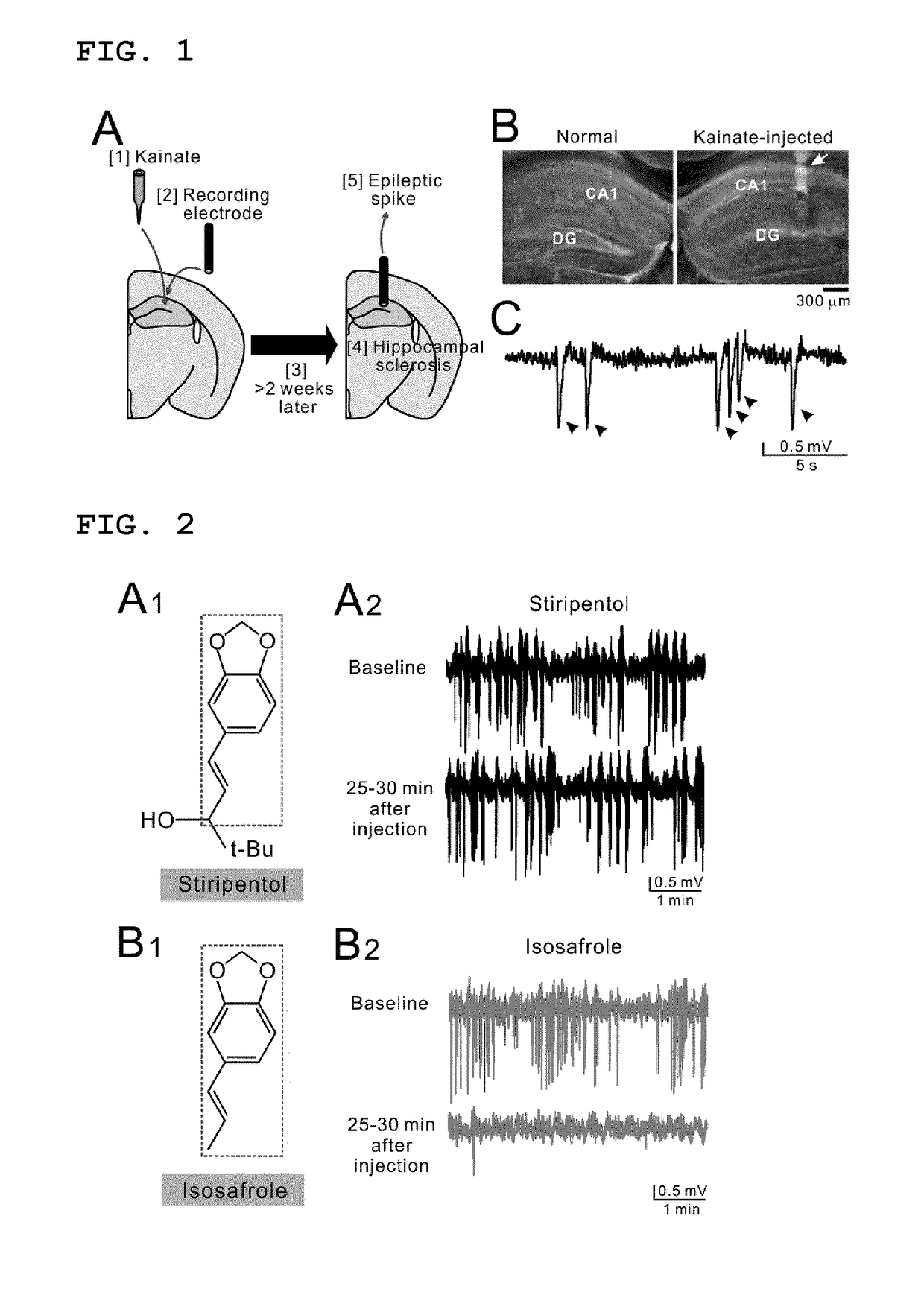
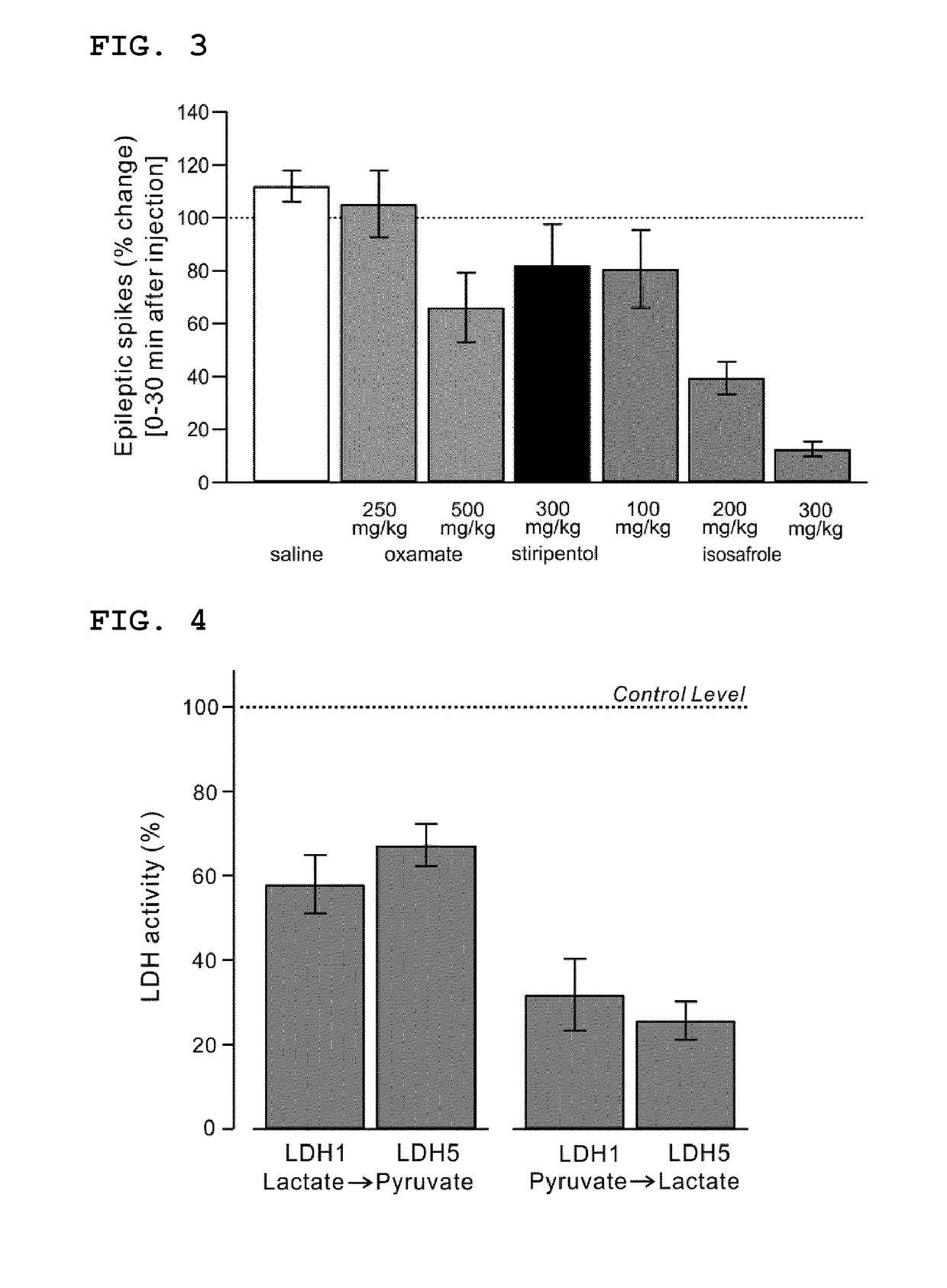
Lactate dehydrogenase inhibitor and antiepileptic drug containing the same
ActiveUS10350192B2Inhibited refractory epilepsyNervous disorderOrganic chemistryLactate dehydrogenaseAntiepileptic drug
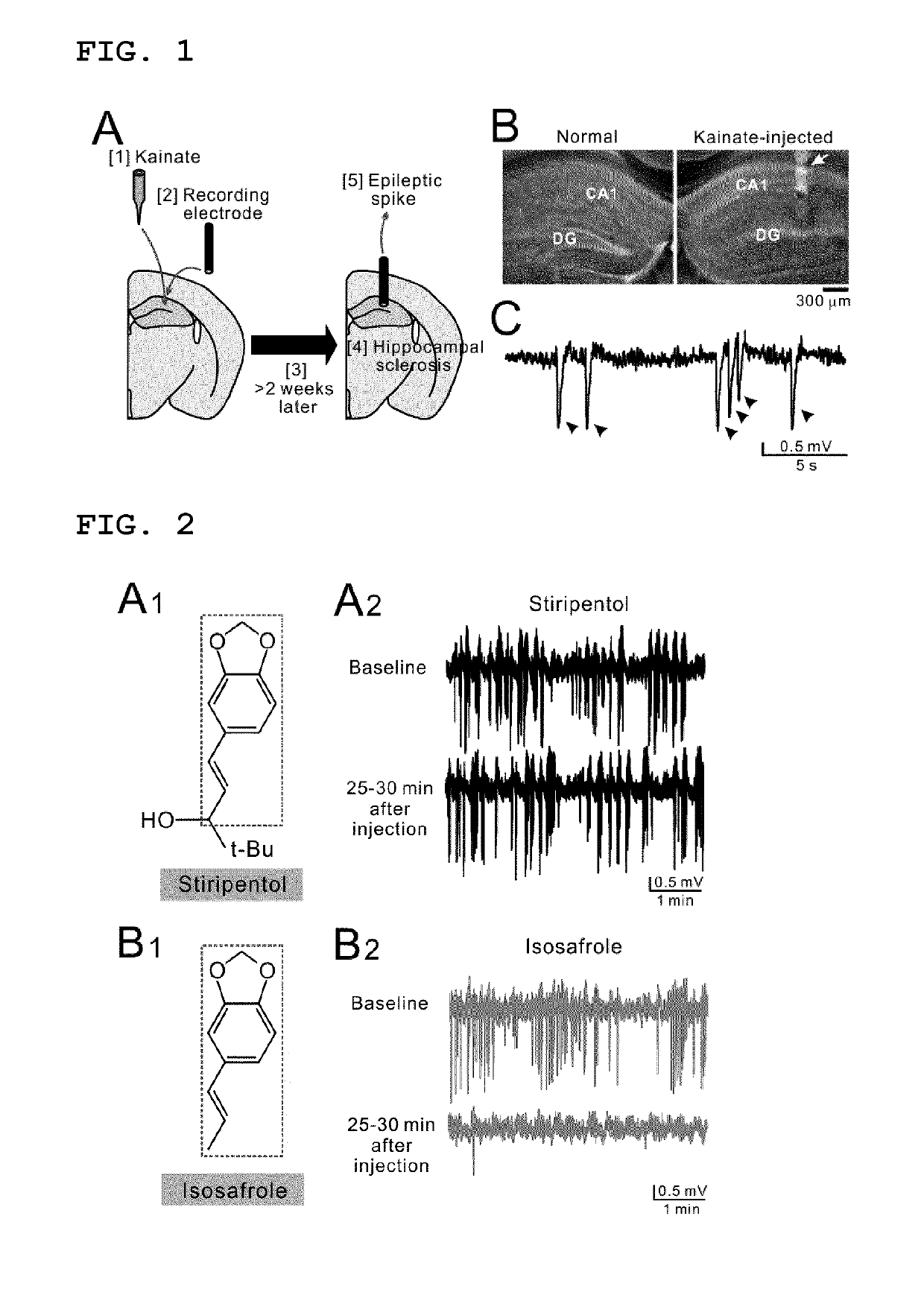
The invention provides a lactate dehydrogenase inhibitor that makes it possible to suppress refractory epilepsy in which conventional antiepileptic drugs are ineffective, and an antiepileptic drug containing said inhibitor. The lactate dehydrogenase inhibitor of the invention contains a compound represented by formula (III); i.e., isosafrole or a compound having isosafrole as a scaffold, and the antiepileptic drug of the invention has these compounds as an active ingredient.
Owner:UNIV OKAYAMA
Pharmaceutical composition and method for treating seizure disorders
PendingUS20210113489A1Reduce dosing frequencyLow variabilityHydroxy compound active ingredientsPharmaceutical non-active ingredientsPharmaceutical drugSeizure frequency
The present disclosure relates to the transdermal administration of cannabidiol (CBD) for the reduction of seizure frequency in the treatment of “treatment-resistant epilepsy” (TRE).
Owner:PIKE THERAPEUTICS INC
Cyclopenthiazide induced novel epilepsy model
InactiveCN101439190AAvoid interferenceInjection of small dosesIn-vivo testing preparationsAnimal husbandryMammalClassification methods
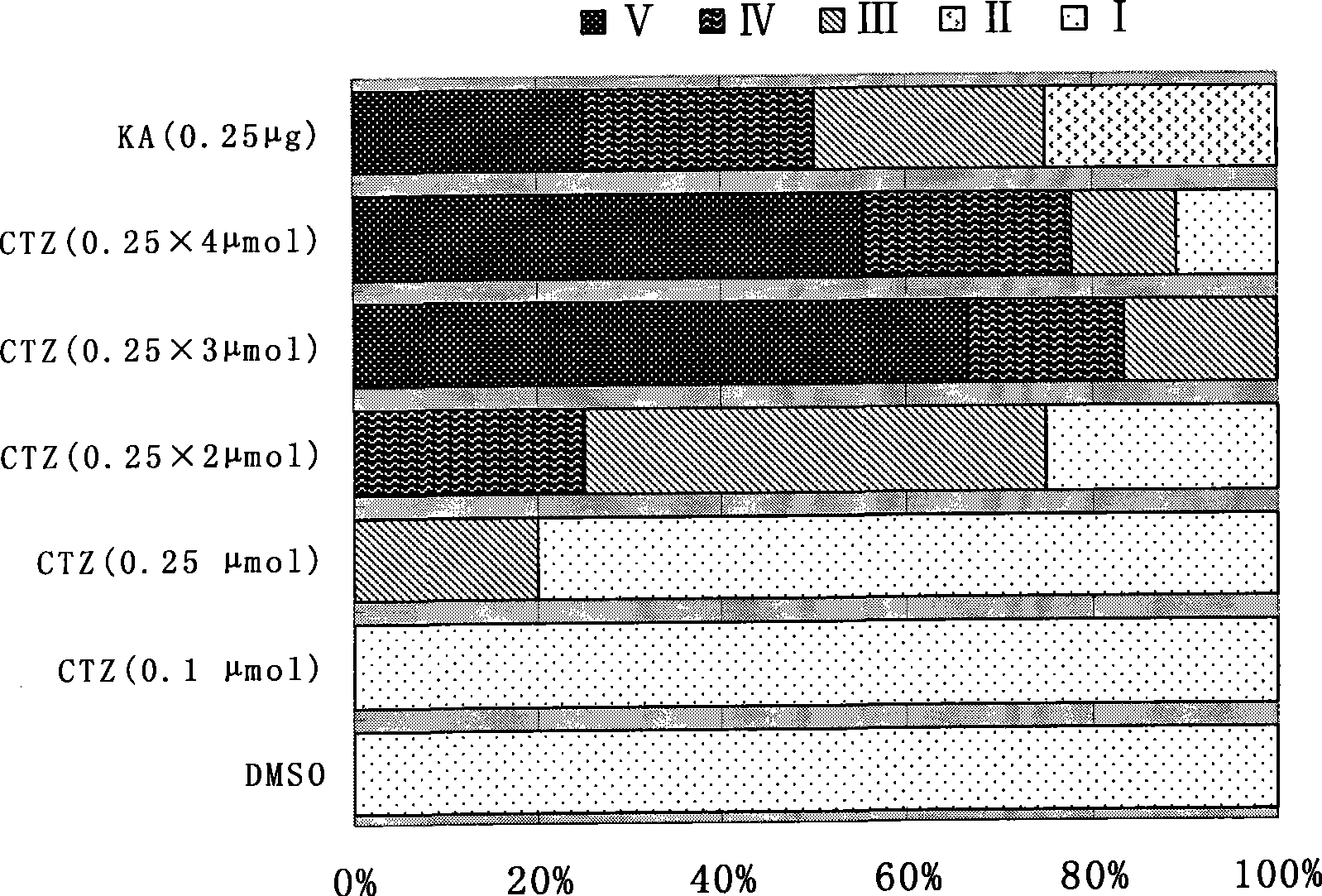
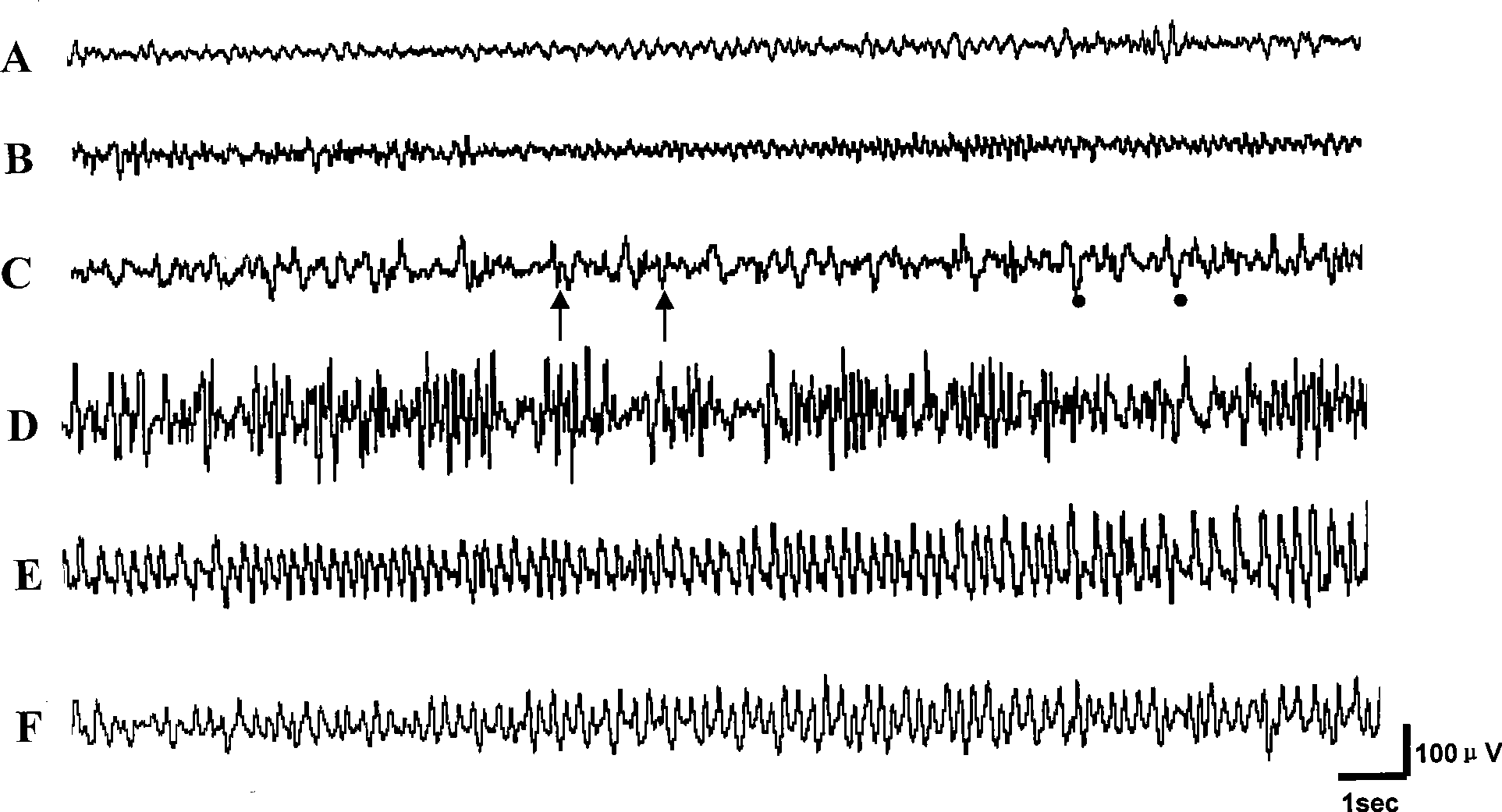
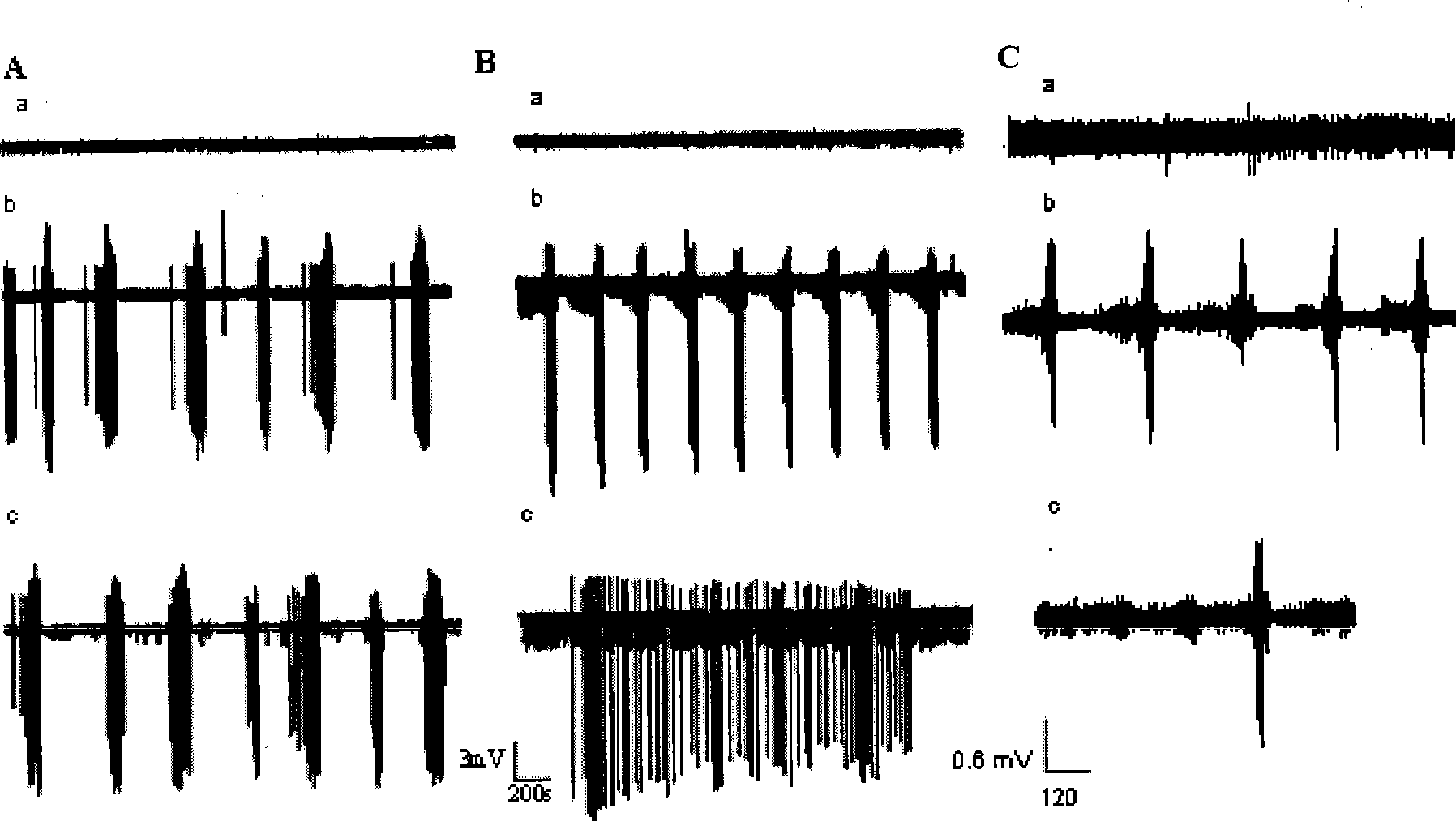
The invention discloses an application of cyclopenthiazide in the preparation of an epilepsy animal model as a drug for inducing epilepsy, the model adopts the cyclopenthiazide for carrying out the local injection of brain of a mammal, the injection is carried out twice or more in 2d-20d with 0.01-0.5 mumol / time, and the total safe effective dose is 0.1-5mumol. A rat appears the II-V-class epilepsy attack of the Racine classification method after the injection, and an EEG simultaneously records the electroencephalogram discharge with the appearance of the epilepsy. The application takes the cyclopenthiazide as an inducer for establishing the new epilepsy animal model, thereby providing a beneficial tool for researching the pathogenesis of the epilepsy, seeking a target point for inhibiting the epilepsy attack and screening the drugs with higher effectiveness for the drug-resistant refractory epilepsy.
Owner:CHONGQING TECH & BUSINESS UNIV
Chinese medicinal composition for treating refractory epilepsy and preparation method thereof
InactiveCN104474303AReasonable prescriptionGood treatment effectNervous disorderAnthropod material medical ingredientsMedicinal herbsGastrodia
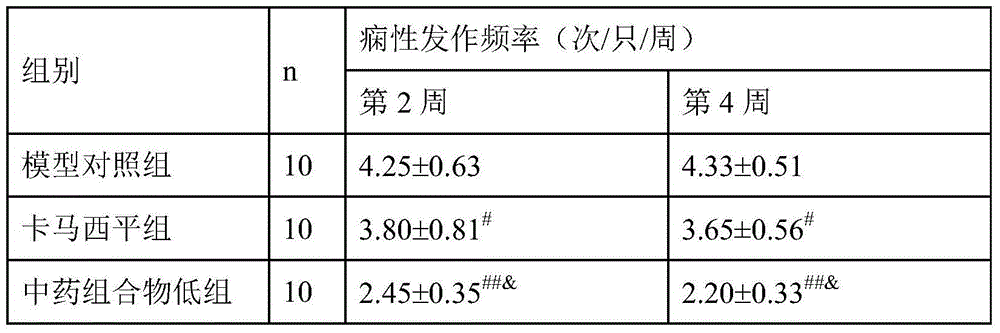
The invention belongs to the field of traditional Chinese medicines, relates to a Chinese medicinal composition for treating refractory epilepsy and a preparation method thereof and aims to solve the problems that the existing medicines for treating refractory epilepsy have poor treatment effects. The Chinese medicinal composition comprises the following Chinese medicinal herbs (components) in parts by weight: 45-60 parts of indianmulberry roots, 5.5-7 parts of tall gastrodia tuber, 30-40 parts of prepared rhizome of adhesive rehmannia, 25-30 parts of milkvetch roots, 30-35 parts of unibract fritillary bulbs, 85-90 parts of white peppers, 15-20 parts of Chinese gall, 12-15 parts of dried tangerine peels, 5-10 parts of Chinese dodder seeds, 9-15 parts of cassia barks, 10-15 parts of sweet wormwood herb, 6-9 parts of capillary wormwood seedlings and 10-15 parts of liquorice roots. The Chinese medicinal composition provided by the invention can be used for treating the behavior of a patient with refractory epilepsy and reducing the incidence of refractory epilepsy and is suitable for clinical popularization and application.
Owner:信洪利
Method for analyzing heart rate variability, apparatus and use thereof
ActiveUS11154255B2Good curative effectPrecise processHealth-index calculationSensorsRR intervalPharmaceutical drug
A method for analyzing heart rate variability, and an apparatus and use thereof, the method for analyzing heart rate variability including collecting ECG data in vitro; digitizing and denoising the ECG data; forming the processed ECG data into a sinus NN interval sequence; selecting sinus NN interval data of 4 hours in an awake state; performing MSE calculation on the sinus NN interval sequence of 4 hours in an awake state; and extracting parameters representing the complexity of a heart rate by using MSE curves. The present invention may provide accurate and efficient screening for drug-refractory epilepsy patients who are suitable for vagus nerve stimulation surgery, thus avoiding unnecessary expenses, and avoiding missing the most opportune moment for treatment. At the same time, patients suitable for VNS surgery are selected by using MSE complexity feature parameters of ECG, thus improving the overall efficacy of VNS therapy.
Owner:BEIJING PINS MEDICAL +1
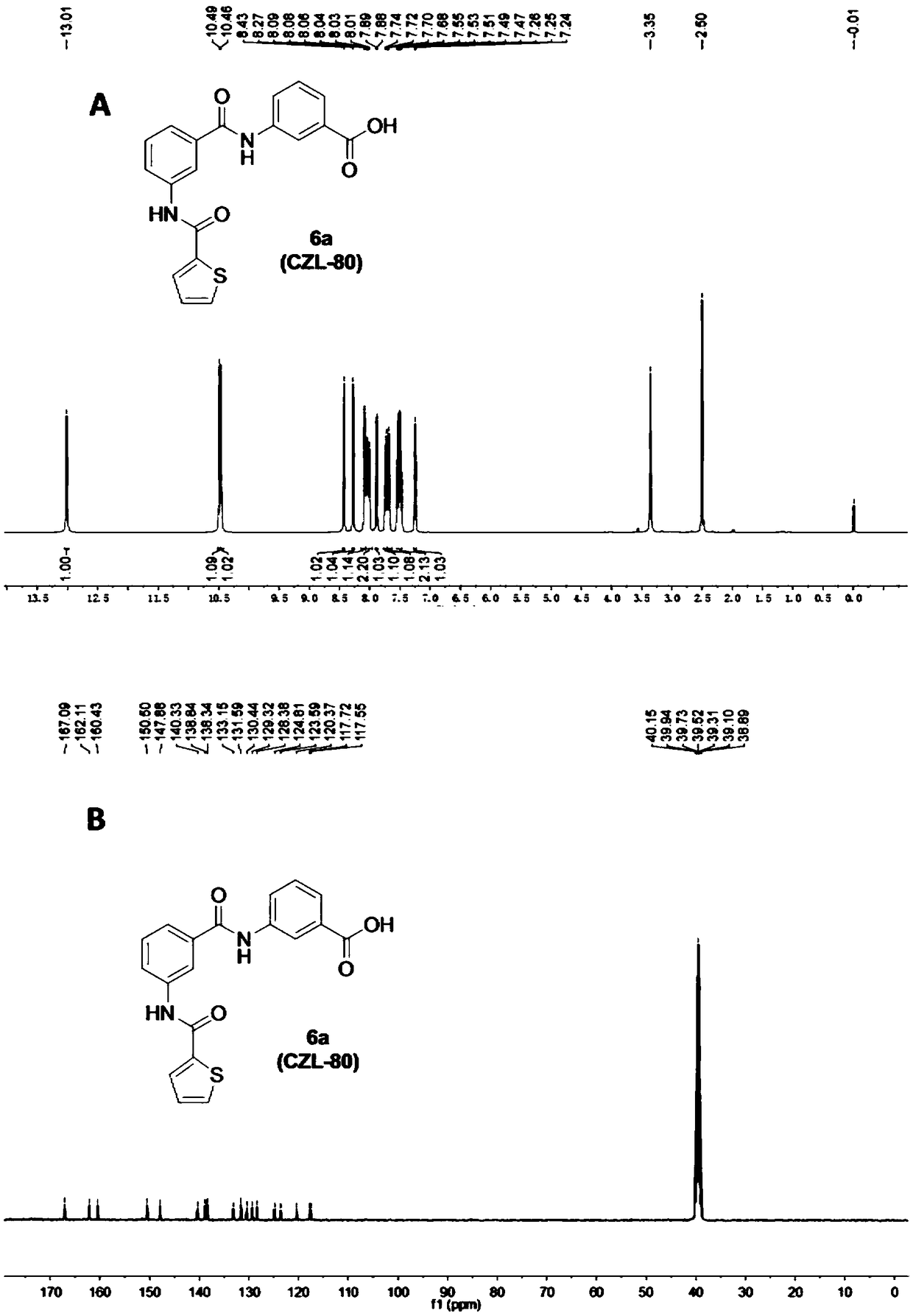
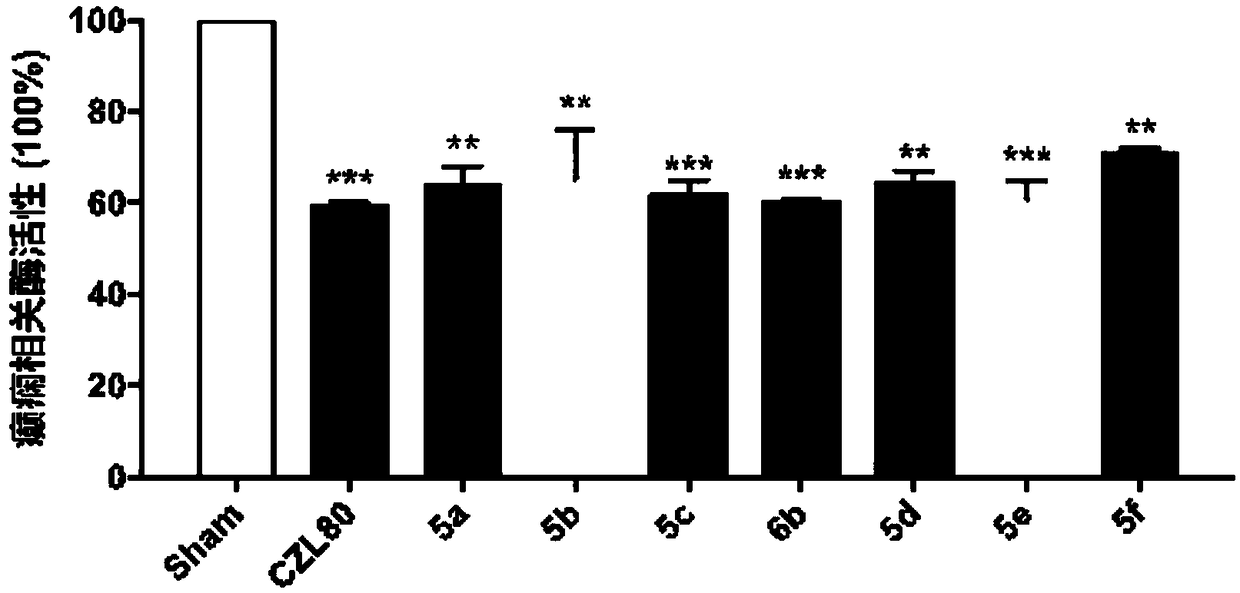
3-acylamino-N-arylbenzamide compound as well as preparation and application thereof
InactiveCN108864038AGood treatment effectReduce the number of spontaneousNervous disorderOrganic chemistryEpilepsy treatmentPentylenetetrazol
The invention provides a 3-acylamino-N-arylbenzamide compound and pharmaceutically acceptable salt thereof. The synthesis of the compound is divided into different synthetic blocks by adopting a convergent synthetic route and a target compound can be quickly prepared in batches through docking combination. The experiment proves that the compound provided by the invention has the advantages that the epileptic seizure latency induced by pentylenetetrazol can be significantly prolonged and the grade and mortality rate of epileptic seizures are reduced; a threshold of epileptic seizure induced bymaximum electroshock can be significantly increased and the mortality rate is reduced; in addition, a seizure threshold of refractory epilepsy can be obviously increased; besides, no obvious toxic orside effects are found, which indicates that the 3-acylamino-N-arylbenzamide compound can relieve or control epileptic seizures or even has a certain therapeutic effect on the refractory epilepsy which cannot be effectively treated by traditional drugs, so that the compound can be applied to the preparation of antiepileptic drugs. The compound disclosed by the invention has the advantages of easily-obtained raw materials for synthesizing, simple operation, high process scalability, suitability for industrial production and capability of providing a novel drug for epilepsy treatment. The structure of a general formula I is shown in the description.
Owner:ZHEJIANG UNIV
Pharmaceutical preparation for treating intractable epilepsy
InactiveCN113018386AStrong targetingDefine Quantitative RequirementsNervous disorderHydroxy compound active ingredientsCannabis sativa plantDisease
The invention discloses a pharmaceutical preparation for treating intractable epilepsy, the preparation comprises a cannabis sativa extract, a gastrodia elata extract, succinylated protein, borneol and an auxiliary agent, the active ingredient of the cannabis sativa extract is a compound with an alkyl resorcinol structure, and the active ingredient of the gastrodia elata extract is a compound with an alkyl resorcinol structure. As an extraction substance which is extracted from a cannabis sativa plant and has the same biological activity, the pharmaceutical preparation has a great synergistic effect on playing a pharmacological effect on refractory epilepsy, and the pharmacological activity of the pharmaceutical preparation is effectively improved; the gastrodia elata extract can provide effective nutrition and repair nerves in the brain, and the borneol can be used as a channel guiding drug to enhance absorption of other drugs in a blood brain barrier and effectively relieve convulsion, spasm, unconsciousness, wandering and other clinical characteristics accompanied by epilepsy disease attack, the succinylated protein in the preparation can prevent the drug from being damaged in an acid environment, and the nano-emulsion preparation prepared by adding the succinylated protein can effectively improve the bioavailability and stability of the drug.
Owner:黑龙江汉普康制药有限公司
Sulfasalazine salt compositions and methods of using the same
InactiveUS20200392084A1Improve solubilityImprove stabilityOrganic active ingredientsNervous disorderDiseaseSulfanilamide
Sulfasalazine salt compositions are provided. In some cases, the sulfasalazine salts have a crystalline form. The subject crystalline sulfasalazine salts can provide a water soluble form of the active compound that finds use in pharmaceutical compositions and therapeutic applications. The subject crystalline sulfasalazine salts can provide increased solubility as compared to the zwitterionic or free acid form of sulfasalazine. Also provided are pharmaceutical compositions including the subject sulfasalazine salt compositions. Methods of treating a neurological related disease such as refractory epilepsy using the subject crystalline sulfasalazine salts and pharmaceutical compositions are also provided.
Owner:ABU IZZA KHAWLA +3
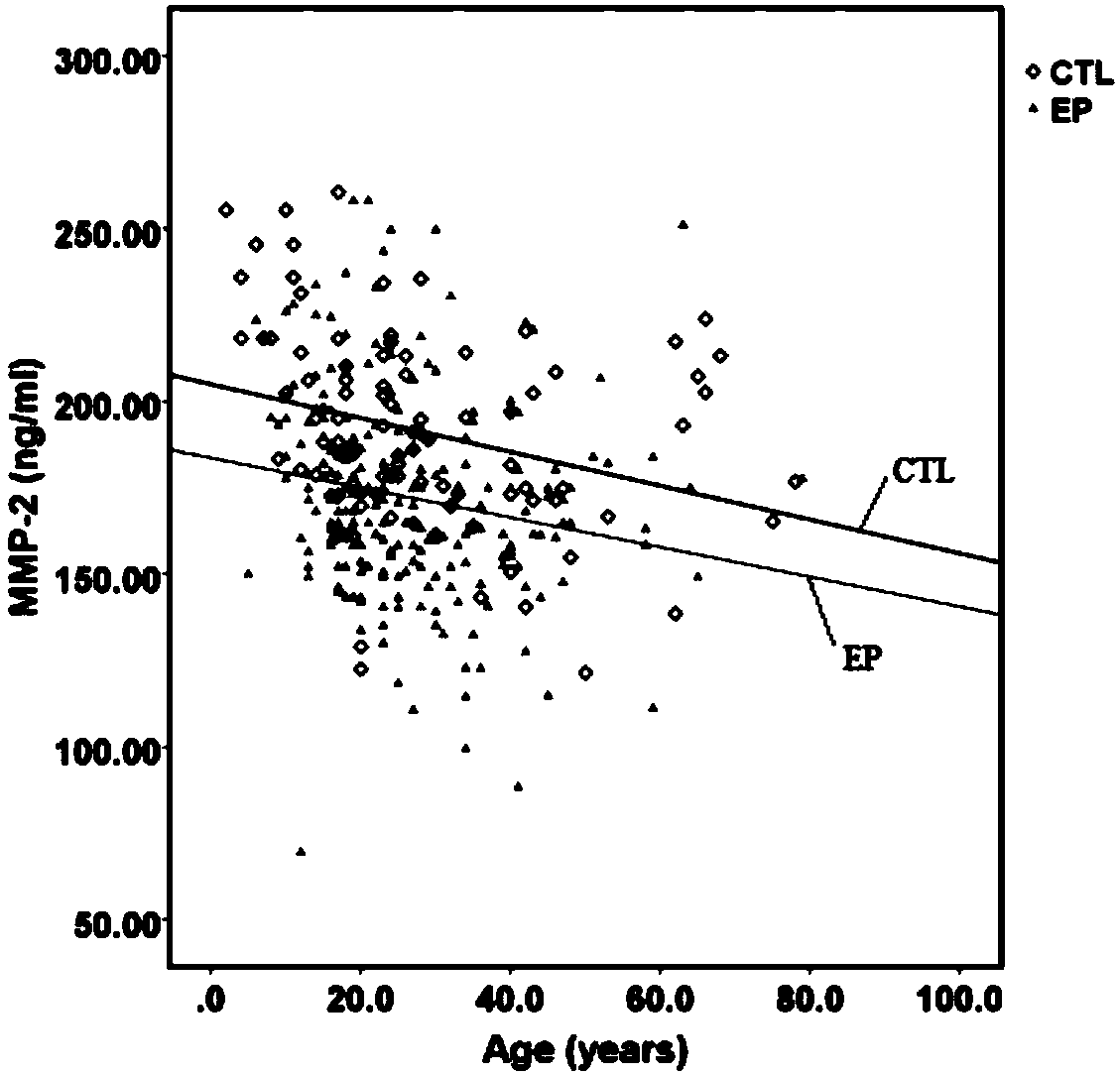
Liquid phase chip kit for detecting refractory epilepsy
InactiveCN109682969AIncreased sensitivityImprove accuracyDisease diagnosisBiological testingMedicineMicrosphere
The invention discloses a liquid phase chip kit for detecting the refractory epilepsy. The kit comprises coated microspheres, biotin labeled detection antibodies, streptomycin R phycoerythrin, a reaction buffer and a reaction dilution solution; the coated microspheres comprise microspheres coated with TIMP-1 capture antibodies, microspheres coated with MMP2 capture antibodies, microspheres coatedwith MMP3 capture antibodies and microspheres coated with MMP9 capture antibodies, and the microspheres coated with different capture antibodies have different color codes; the detection antibodies include a TIMP-1 detection antibody, an MMP 2 detection antibody, an MMP 3 detection antibody and an MMP 9 detection antibody; the reaction buffer is a weakly alkaline PBS buffer; a dilution buffer is aPBS buffer containing 0.05wt%-0.15wt% BSA and has a pH value of 7.0-7.5. By using the kit, the purpose of timely discovery and accurate diagnosis of the epilepsy can be achieved.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Medicine for treating intractable epilepsy and preparation method thereof
InactiveCN113018387APromote absorptionGood synergyNervous disorderHydroxy compound active ingredientsCannabisDisease
The invention discloses a medicine for treating intractable epilepsy and a preparation method thereof, the medicine comprises a cannabis sativa extract, a gastrodia elata extract and borneol, the effective component of the cannabis sativa extract is a compound which is separated and extracted from cannabis sativa and has an alkylresorcinol structure, the medicine has a great synergistic effect on playing a pharmacological effect on intractable epilepsy, and the pharmacological activity of the pharmaceutical composition is effectively improved; the gastrodia elata extract can provide effective nutrition and repair nerves in the brain, the borneol can be used as a channel guiding drug to enhance absorption of other drugs in a blood brain barrier and effectively relieve convulsion, spasm, unconsciousness, wandering and other clinical characteristics accompanied by epilepsy disease attack, and the three drug components are subjected to traditional Chinese medicine prescription compatibility, and the intractable epilepsy is effectively treated in a targeted manner from three aspects of pathological treatment of epilepsy diseases, nutrition and repair of damaged nerves in the brain and relieving of clinical characterization.
Owner:黑龙江汉普康制药有限公司
Lactate dehydrogenase inhibitor and antiepileptic drug containing the same
ActiveUS20180015068A1Inhibited refractory epilepsyNervous disorderOrganic chemistryAntiepileptic AgentsDepressant
The invention provides a lactate dehydrogenase inhibitor that makes it possible to suppress refractory epilepsy in which conventional antiepileptic drugs are ineffective, and an antiepileptic drug containing said inhibitor. The lactate dehydrogenase inhibitor of the invention contains a compound represented by formula (III); i.e., isosafrole or a compound having isosafrole as a scaffold, and the antiepileptic drug of the invention has these compounds as an active ingredient.
Owner:UNIV OKAYAMA
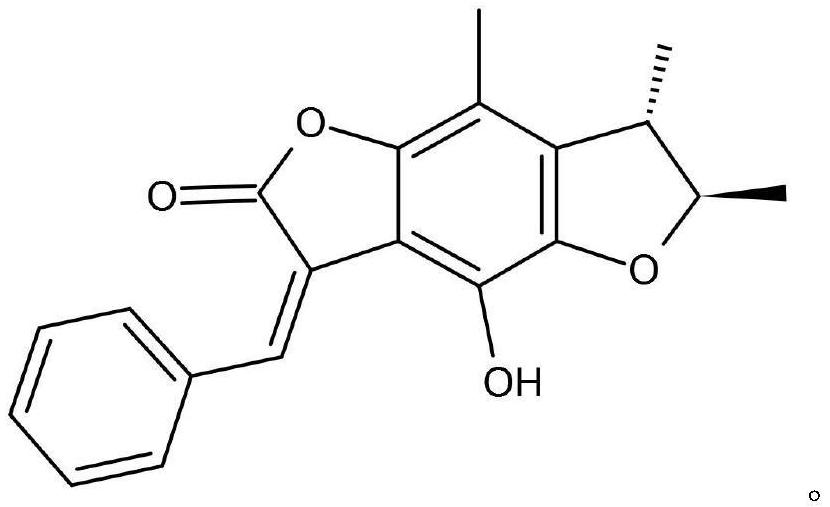
Application of benzodifuranone compound in treatment of intractable epilepsy
ActiveCN113679714AGood synergyNervous disorderAnhydride/acid/halide active ingredientsDiseasePharmaceutical drug
The invention discloses application of a benzodifuranone compound in preparation of medicines for treating intractable epilepsy, and belongs to the technical field of medicines. According to the application, the benzodifuranone compound is used for treating epilepsy for the first time, and particularly a new candidate drug is provided for treatment of intractable epilepsy. In addition, the invention further provides a compound medicine combining the benzodifuranone compound and sodium valproate, and the benzodifuranone compound and the sodium valproate have an obvious synergistic effect in treatment of intractable epilepsy diseases.
Owner:AFFILIATED HOSPITAL OF JINING MEDICAL UNIV
Application of ethambutol hydrochloride in the treatment of refractory epilepsy
ActiveCN109999016BAdvantages and Notable ImprovementsHas antiepileptic effectOrganic active ingredientsNervous disorderNew medicationsUse medication
The invention discloses the application of ethambutol or a pharmaceutically acceptable salt thereof as an active ingredient in the preparation of a drug for treating intractable epilepsy, and belongs to the technical field of medicine. Because the dosage of ethambutol hydrochloride is low, the toxic and side effects must be reduced, thereby reducing the toxic and side effects of the patient's medication. The new anti-refractory epilepsy drug has very significant social and economic significance.
Owner:浙江药苑生物科技有限公司
Pharmaceutical composition for treating epilepsy
ActiveCN113288958AAccelerated deathHigh riskNervous disorderAnthropod material medical ingredientsSide effectCognitive impairment
The invention discloses a pharmaceutical composition for treating epilepsy. The pharmaceutical composition is prepared from the following raw materials in parts by weight: 10-20 parts of radix bupleuri, 14-26 parts of rhizoma acori graminei, 21-39 parts of uncaria, 21-39 parts of fossil fragments, 21-39 parts of oyster, 21-39 parts of white paeony root, 7-13 parts of divaricate saposhnikovia root, 10-20 parts of fried stiff silkworm, 10-20 parts of earthworm and 3-7 parts of liquorice. The composition disclosed by the invention has a remarkable effect on treating epilepsy, also has the effects of improving symptoms and reducing or controlling attack on part of intractable epilepsy, and can play a synergistic effect with anti-epilepsy western medicines when being used for an epileptic state, and meanwhile, can promote a patient to wake up. Compared with western medicines, the composition disclosed by the invention is safer, has no obvious toxic and side effects in long-term clinical application, has no side effects such as cognitive impairment, language impairment, dizziness and the like, has no teratogenesis effect, and has a clinical application prospect.
Owner:TEACHING HOSPITAL OF CHENGDU UNIV OF T C M
A drug that reverses drug resistance in refractory epilepsy
ActiveCN105483130BReduce pump rateReverse drug resistanceNervous disorderGene therapyLentivirusNucleotide sequencing
The invention discloses a nucleotide sequence shown in SEQ ID NO.1, and further discloses a recombinant virus containing the nucleotide sequence and application thereof. The recombinant lentivirus can inhibit expression of drug resistance protein Pgp and a drug-resistance related gene HIF-1alpha and also can decrease the pumping rate of a drug, can reverse the drug resistance of intractable epilepsy, can be jointly used with other drugs for treating epilepsy to treat the intractable epilepsy and is good in clinical application prospect.
Owner:WEST CHINA HOSPITAL SICHUAN UNIV
Construction method and application of zebra fish intractable epilepsy model
InactiveCN111304251ABig foundationGreat application value in clinical researchMicroinjection basedVector-based foreign material introductionGABRG2 geneAntiepileptic drug
The invention discloses a construction method of a zebra fish intractable epilepsy model. A mutant human GABRG2 gene of which the 282th proline codon is mutated into serine is transferred into a zebrafish, so that the zebra fish becomes a zebra fish line with intractable epilepsy phenotype. The method provides an effective method basis for construction of the zebra fish intractable epilepsy model, also provides an important tool for epilepsy pathology research and antiepileptic drug screening research, and has great foundation and clinical research application value.
Owner:NANTONG UNIVERSITY
Methods for the Prevention or Treatment of Epilepsy
InactiveUS20190269752A1Induce remissionNervous disorderPeptide/protein ingredientsReceptor Tyrosine Kinase BRefractory epilepsy
The present disclosure relates to methods of preventing or treating epilepsy comprising administering a receptor tyrosine kinase B (TrkB) inhibitor. In particular, the present disclosure relates to methods of treating a subject susceptible to the development of epilepsy, methods of inducing remission of epilepsy in a subject, and methods of transforming medically refractory epilepsy in a subject to medically responsive epilepsy comprising administering a therapeutically effective amount of a TrkB inhibitor or a phospholipase Cγ1 (PLCγ1) inhibitor.
Owner:DUKE UNIV
A drug for intractable epilepsy
ActiveCN104288231BReduce dosageReach the anti-epileptic effect of Chinese and Western medicineOrganic active ingredientsNervous disorderClinical efficacyRegimen
The invention provides a medicine for treating refractory epilepsy. The medicine is a preparation prepared from the following raw materials in parts by weight: 45-62 parts of Phenobarbital, 125-153 parts of potassium bromide, 5.5-12.6 parts of valerian extract, 6.8-11.5 parts of camphor, 1.5-3.5 parts of fried nux vomica powder, 80-120 parts of calcium gluconate, 80-120 parts of calcium carbonate; and the medicinal auxiliary materials comprise a disintegrating agent and a lubricating agent, wherein the disintegrating agent accounts for 20-80 percent of the total mass of the raw materials, and the lubricating agent accounts for 2-10 percent of the total mass of the raw materials. The preparation method comprises the following steps: performing valerian extract pretreatment and preparing the medicine for treating the refractory epilepsy. The medicine produced by the invention can achieve the effects of obviously improving the anti-epileptic curative effect, shortening the course of treatment, rapidly improving the symptoms, relieving the pain and reducing the use amount of Western medicines, the adverse reactions are obviously lower than those of the Western medicines, and the satisfied clinical curative effect is achieved.
Owner:AFFILIATED RENHE HOSPITAL OF CHINA THREE GORGES UNIV
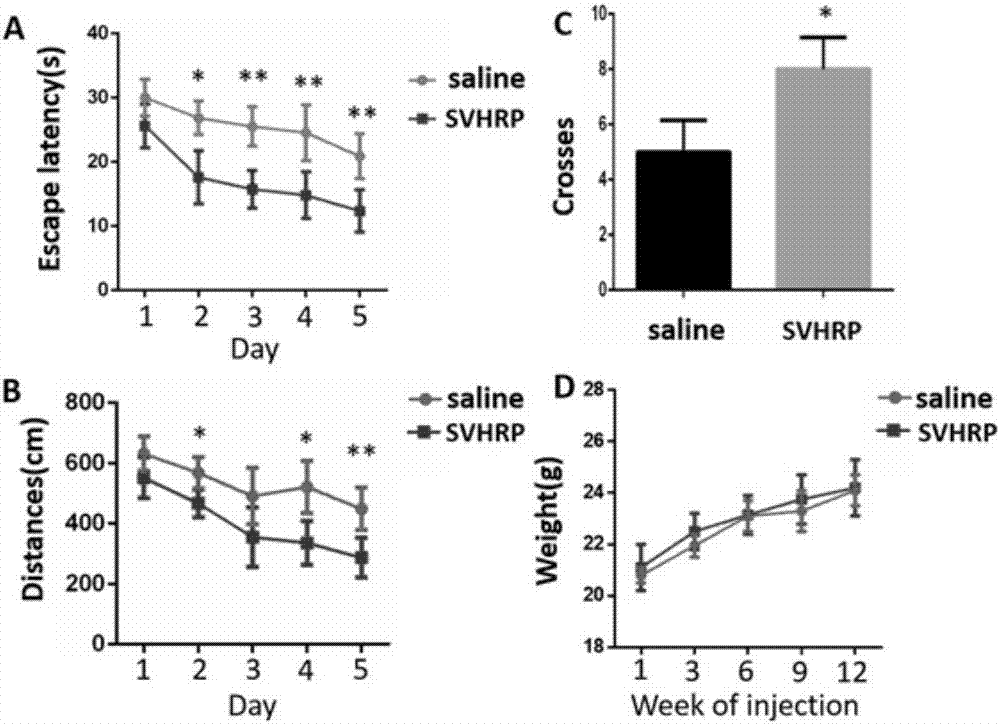
A kind of scorpion venom heat-resistant synthetic peptide and use thereof
ActiveCN106220713BHas a promoting effectImprove chemotaxis abnormalitiesNervous disorderPeptide/protein ingredientsChemical synthesisScorpion Venoms
The invention discloses a scorpion venom heat-resistant synthetic peptide and its application, belonging to the field of polypeptide drug research and development. The polypeptide of the present invention is the amino acid sequence of the scorpion venom heat-resistant peptide (SVHRP) detected from the venom of the traditional Chinese medicine East Asian scorpion scorpion (BmK). A sample of the heat-resistant peptide extract from scorpion venom with pharmacodynamic activity of Alzheimer's disease (premature Alzheimer's disease) was separated by LaGM composite material and repeated rapid magnetic separation, and then performed parallel mass spectrometry using nanoliter reversed-phase chromatography-electrospray mass spectrometry (nanoLC-ESI-MS). A polypeptide sequence consisting of 15 amino acid residues was detected in the experiment; a heat-resistant synthetic peptide of scorpion venom was prepared through solid-phase chemical synthesis, chromatographic purification and mass spectrometry identification. Its amino acid sequence is shown in SEQIDNo 1, which maintains the The pharmacodynamic activity and safety of the scorpion venom heat-resistant peptide, and the bioactivity of the scorpion venom heat-resistant peptide also promotes the reverse differentiation of glial cells into neural stem cells.
Owner:上海万锦医药科技有限公司
A screening kit for intractable epilepsy
ActiveCN104762412BLow risk of developing treatment-resistant epilepsyReduce riskMicrobiological testing/measurementCvd riskRefractory epilepsy
The invention discloses a screening reagent box for intractable epilepsy. The screening reagent box for the intractable epilepsy comprises an optional reagent used for detecting the expression level of micro RNA-153. The invention further discloses an application of the optional reagent used for detecting the expression level of the micro RNA-153 in preparing a reagent used for screening the intractable epilepsy. According to the reagent box, the expression level of the micro RNA-153 is detected, the risk of suffering from the intractable epilepsy for crowds to be examined can be judged, the reagent box can be used for auxiliary diagnosis of clinical intractable epilepsy, an effective base is provided for a patient to take relevant treatment measures or decisions, and a good clinical application prospect is achieved.
Owner:SICHUAN CREDIT PHARMA
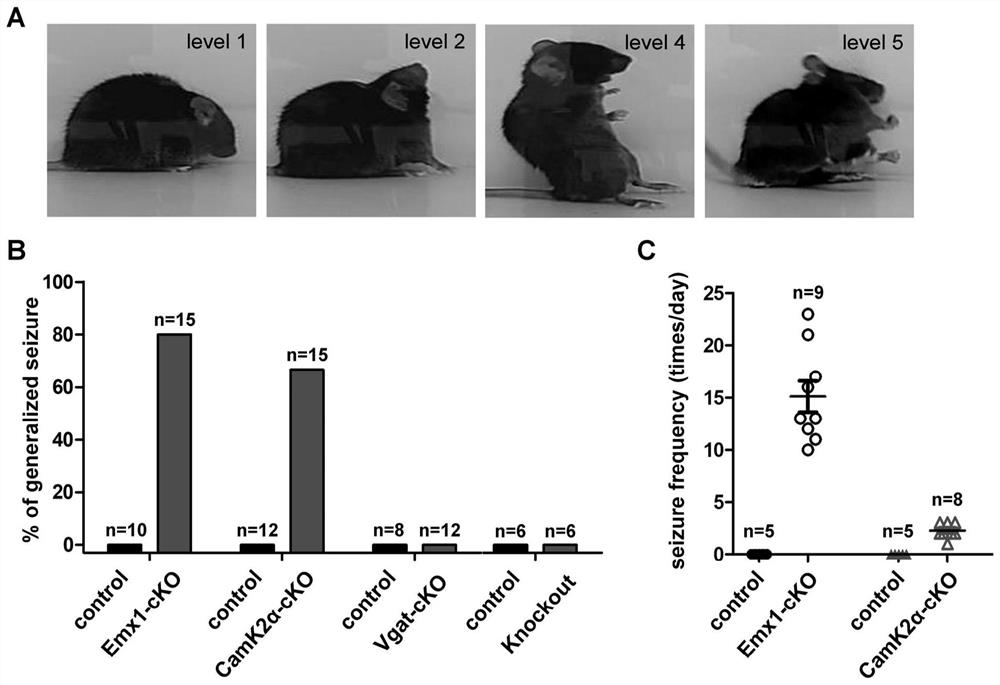
Preparation and application of epilepsy animal model
The invention provides preparation and application of an intractable epilepsy animal model. Specifically, the invention provides a preparation method of an intractable epilepsy animal model of a non-human mammal, and the preparation method comprises the following steps: (1) providing a non-human mammal A and a non-human mammal B expressed by neuron cell specific Cre recombinase of the same species; wherein the genome of the non-human mammal A has (E1) an endogenous Cdkl5 gene and (E2) a conditional knockout element which is operatively connected with the Cdkl5 gene and is used for conditional knockout of the Cdkl5 gene, and the conditional knockout element is used for conditional knockout of the Cdkl5 gene of the genome of neuronal cells in the presence of the Cre recombinase, so that the Cdkl5 gene is inactivated; (2) carrying out mating breeding on the animal A and an animal B to obtain a filial generation non-human mammal C of which the Cdkl5 gene is specifically knocked out in neuronal cells; and (3) culturing the filial generation non-human mammal C to obtain the intractable epilepsy animal model. According to the invention, the curative effect of the anti-epilepsy drug can be preliminarily evaluated according to the change of spontaneous epilepsy phenotype of animals.
Owner:CENT FOR EXCELLENCE IN BRAIN SCI & INTELLIGENCE TECH CHINESE ACAD OF SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com