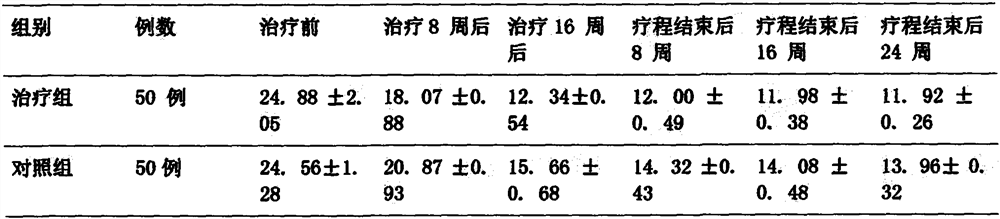
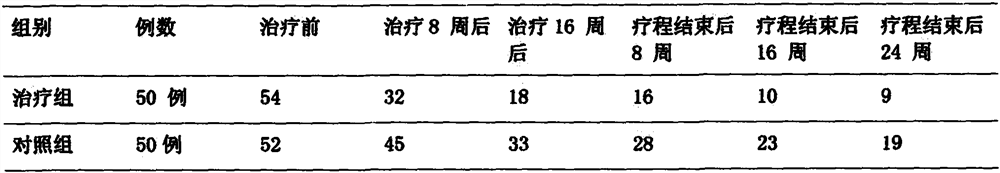
Pharmaceutical preparation for treating intractable epilepsy
A technology for refractory epilepsy and pharmaceutical preparations, applied in the field of medicine, can solve the problems of inability to complete the consistency of absorption and metabolism time, inability to exert synergistic effects, increasing uncertainty in clinical safety, etc., to achieve nutrition and restoration of clinical manifestations. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A pharmaceutical preparation for the treatment of refractory epilepsy, including 10% of cannabis extract, 5% of Gastrodia elata extract, 0.8% of borneol, 7% of succinylated protein, 20% of sesame oil, and 14% of olive oil in weight percentage %, ethanol 10%, polyethylene glycol 17%, deionized water 16.2%;
[0054] Prepared by the following preparation method, comprising the following steps:
[0055] Step S1, preparing succinylated protein;
[0056] Step S2, taking the formula amount of sesame oil, olive oil, ethanol and polyethylene glycol and mixing evenly, adding the formula amount of cannabis extract, Gastrodia elata extract, succinylated protein and borneol under magnetic stirring conditions to obtain a mixed solution;
[0057] Step S3, adding a formulated amount of deionized water to the mixed solution, performing high-shear treatment to obtain a homogeneous mixed solution;
[0058] In step S4, homogenize the prepared homogeneous mixed solution through a high-pre...
Embodiment 2
[0064] A pharmaceutical preparation for the treatment of intractable epilepsy, including 10% of cannabis extract, 6% of Gastrodia elata extract, 1% of borneol, 13% of succinylated protein, 14% of sesame oil, and 15% of soybean oil in weight percentage %, propylene glycol 14%, glyceride 20%, deionized water 7%;
[0065] Prepared by the following preparation method, comprising the following steps:
[0066] Step S1, preparing succinylated protein;
[0067] Step S2, taking the formula amount of sesame oil, soybean oil, propylene glycol and glyceride and mixing evenly, adding the formula amount of cannabis extract, Gastrodia elata extract, succinylated protein and borneol under magnetic stirring conditions to obtain a mixed solution;
[0068] Step S3, adding a formulated amount of deionized water to the mixed solution, performing high-shear treatment to obtain a homogeneous mixed solution;
[0069] In step S4, homogenize the prepared homogeneous mixed solution through a high-pressu...
Embodiment 3
[0075] A pharmaceutical preparation for treating refractory epilepsy, comprising 5% cannabis extract, 1% Gastrodia elata extract, 0.3% borneol, 4% succinylated protein, 2% castor oil, and coconut oil by weight percentage 2%, corn oil 2%, cottonseed oil 2%, evening primrose oil 2%, cod liver oil 2%, linseed oil 2%, mineral oil 2%, olive oil 2%, peanut oil 2%, soybean oil 2%, Sesame Oil 1%, Pine Nut Oil 1%, Wheat Germ Oil 1%, Squalene 1%, Methyl Caprate 1%, Oleic Acid 1%, Ethyl Oleate 1%, Brucea Brucea Oil 1%, Diethylene Glycol Monoethyl ether 2%, lecithin 2%, deionized water 55.7%;
[0076] Prepared by the following preparation method, comprising the following steps:
[0077] Step S1, preparing succinylated protein;
[0078] Step S2, taking the formula amount of castor oil, coconut oil, corn oil, cottonseed oil, evening primrose oil, cod liver oil, linseed oil, mineral oil, olive oil, peanut oil, soybean oil, sesame oil, pine nut oil, wheat germ oil, Squalene, methyl caprate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com