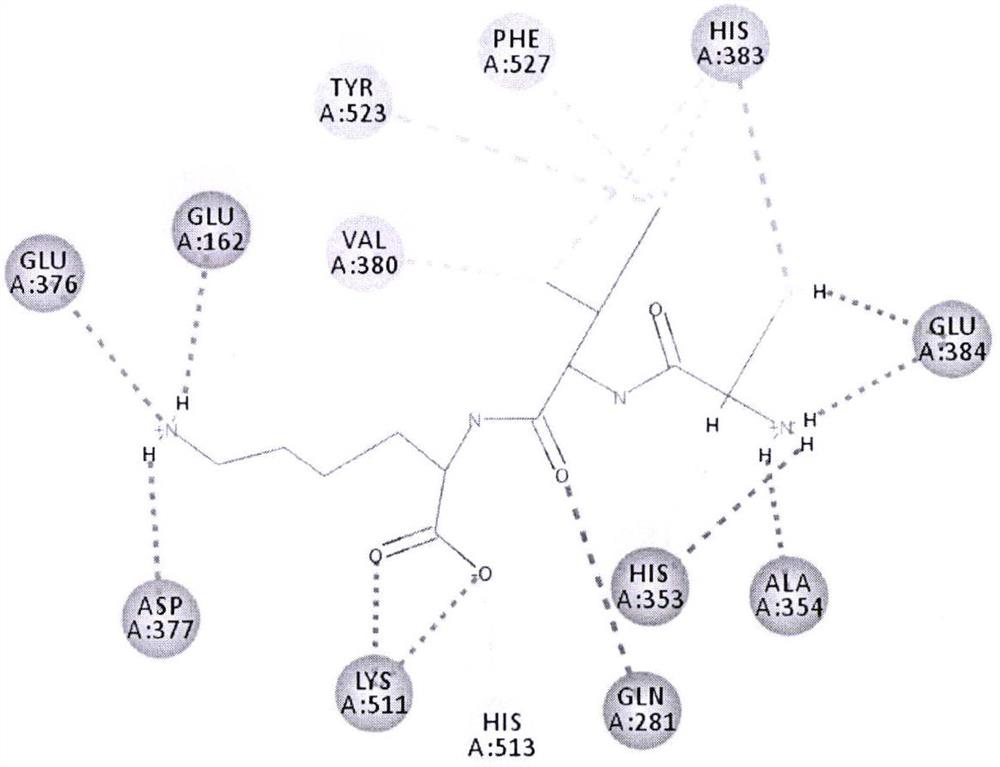
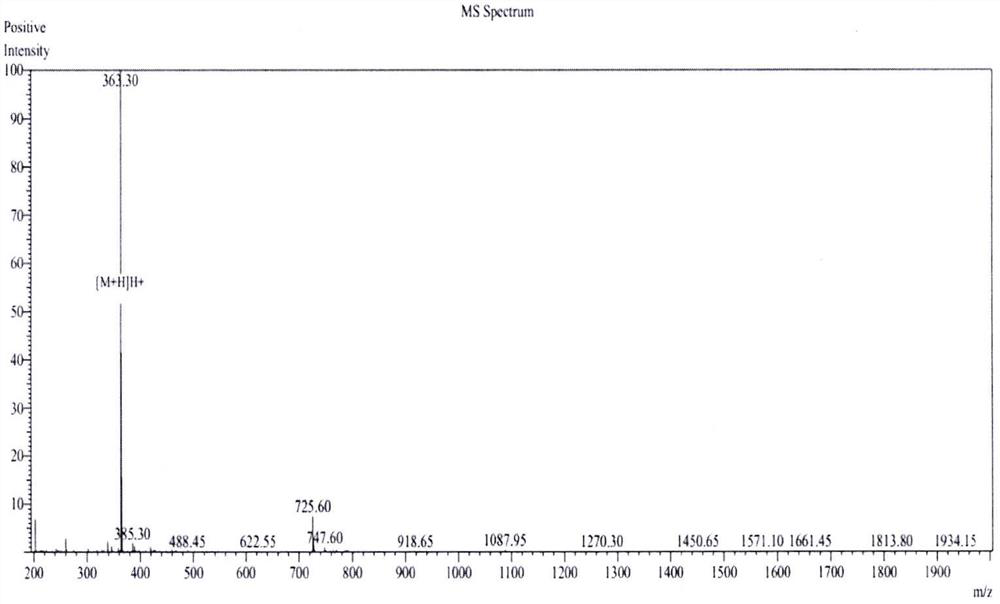
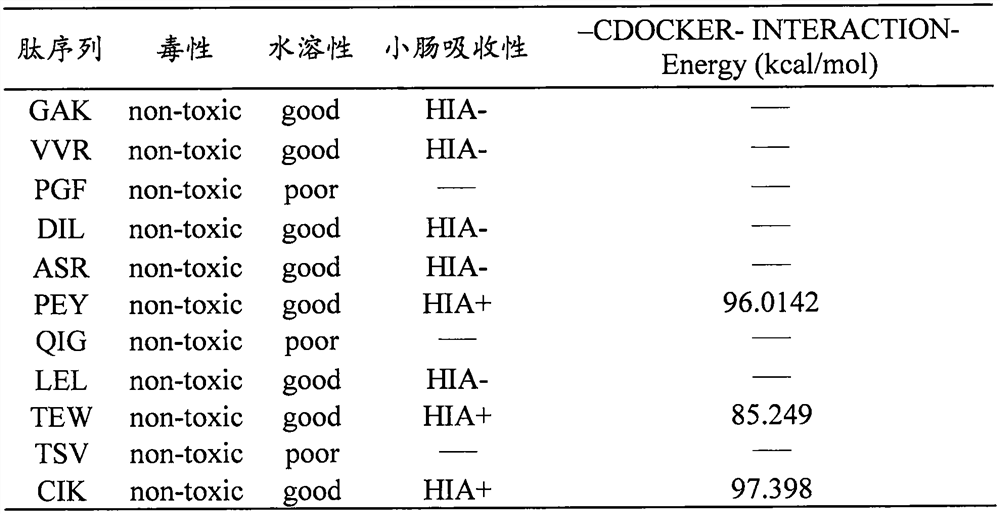
an ace inhibitory peptide
A technology of inhibitory peptides and adjuvant therapy, applied in the field of ACE inhibitory peptides, can solve the problems of complex process, difficulty in obtaining high-purity peptides, and long time consumption, and achieve low cost effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1 Simulated enzyme digestion and virtual screening of ovalbumin
[0018] 1. Virtual gastrointestinal enzymatic hydrolysis of ovalbumin.
[0019] Pepsin (EC 3.4.23.1), trypsin (EC 3.4.21.4) and chymotrypsin (EC 3.4.21.1) were selected to pass through the ExPASy PeptideCutter (http: / / web.expasy.org / peptide_cutter / ) with egg pairs. The albumin sequence (Accession of NCBI: 0705172A) was subjected to simulated digestion to obtain 104 peptide sequences. The tripeptide sequence was compared with the known ACE inhibitory peptides in the BIOPEP-UWM database (http: / / www.uwm.edu.pl / biochemia / index.php / en / biopep) to obtain 11 unreported tripeptide.
[0020] 2. Prediction of toxicity, water solubility and ADME properties.
[0021] Using the online tools ToxinPred (http: / / crdd.osdd.net / raghava / / toxinpred / ), peptide property calculator (http: / / www.innovagen.com / ) and admetSAR (http: / / lmmd.ecust.edu. cn / admetsarl / ) to predict the toxicity, solubility and ADME properties of ...
Embodiment 2 3
[0028] Example 2 Identification of Tripeptide CIK ACE Inhibitory Activity in Vitro
[0029] The ACE inhibitory activity of CIK was determined by HPLC. Take hippuryl-histidyl-leucine (HHL) substrate solution, add CIK solution and mix evenly, preheat in a constant temperature water bath at 37°C for 3-5 minutes, then add ACE solution and mix thoroughly, keep warm at 37°C for 30 minutes, 1mol / L HCl was added to terminate the reaction to obtain a reaction solution. At the same time, boric acid buffer solution was used instead of inhibitor solution as blank control group. The reaction solution was directly analyzed by HPLC system.
[0030] Chromatographic conditions: column temperature 25°C, flow rate 0.5mL / min, mobile phase acetonitrile / water 25:75 isocratic elution, detection wavelength 228nm.
[0031] The experimental results showed that the tripeptide CIK can effectively inhibit the activity of ACE, IC 50 The value was 161 μM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com