Application of MBOAT1 gene in preeclampsia
A preeclampsia, genetic technology, applied in the field of biomedicine, can solve the problems of mother and child harm, not very clear and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
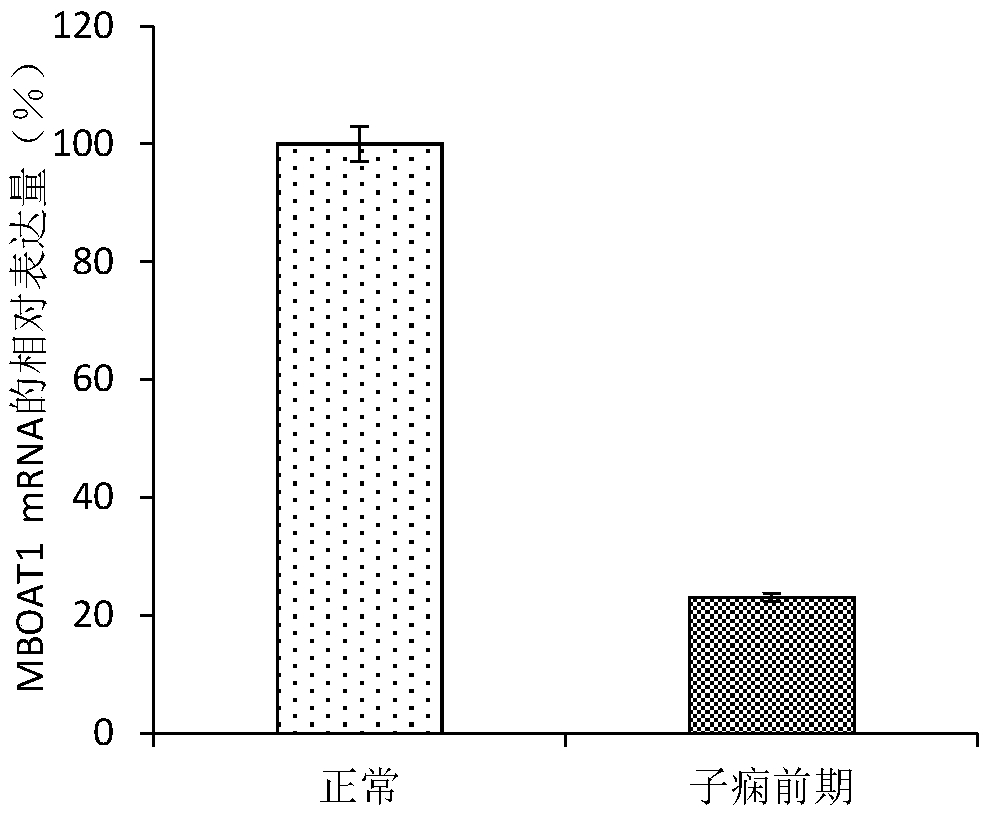
[0053] Example 1 Screening for Abnormally Expressed Genes in the Placental Tissue of Patients with Preeclampsia
[0054] 1. Sample collection
[0055] The placenta tissues of 43 cases of preeclampsia pregnant women and normal pregnant women were collected respectively, rinsed with normal saline twice, after removing water, they were divided into cryopreservation tubes, and stored at -80°C for later use.
[0056] Placental tissue excluded multiple pregnancy, infectious diseases, chemical drug dependence, maternal smoking, fetal congenital malformations and other pregnancy complications and complications, and all included subjects signed an informed consent form before collecting specimens. All the above specimens were obtained with the consent of the organizational ethics committee. Six samples were taken from each group for detection and analysis of gene expression profiles, screening of differentially expressed genes, and verification experiments were carried out in all 43 s...
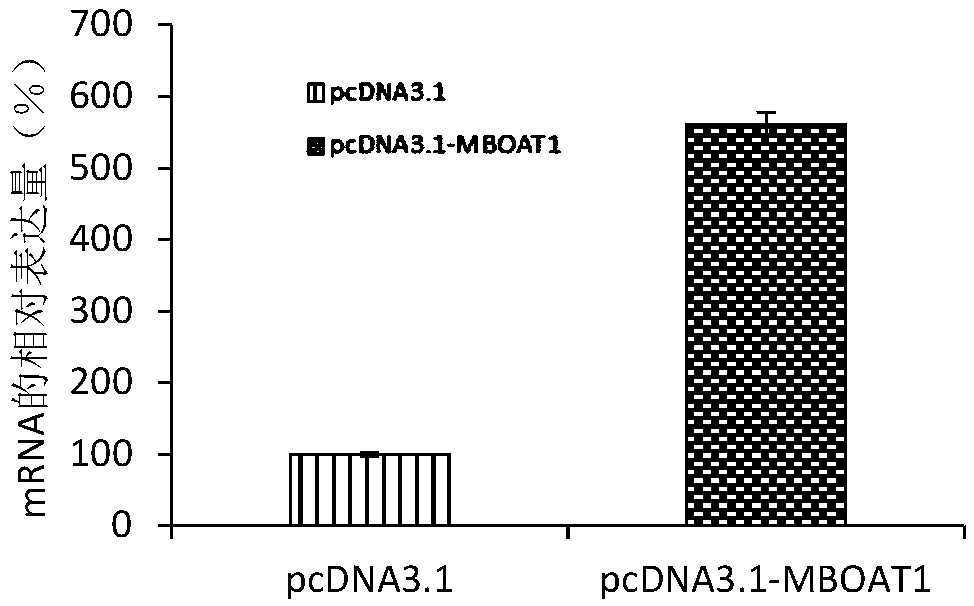
Embodiment 2
[0071] Example 2 QPCR sequencing to verify the differential expression of the MBOAT1 gene
[0072] 1. Large-sample QPCR verification of differential expression of MBOAT1 gene.
[0073] 2. RNA extraction
[0074] Use QIAGEN tissue RNA extraction kit to extract total RNA in embryonic tissue, please refer to the manual for specific steps.
[0075] 3. Reverse transcription:
[0076] 1) Add dNTP mixture 1μl, Oligo dT primer 1μl, total RNA 2μg, add RNase FreeddH 2 O Make the total volume to 10 μl, carry out denaturation and annealing reaction on the PCR instrument, 65°C, 5min, place at 4°C after the reaction is completed.
[0077] 2) Construct a 20 μl reaction system, continue to add 4 μl of 5×Primer Script Buffer, 0.5 μl of RNase Inhibitor, 0.5 μl of Prime Script RTase, RNase Free dd H 2 O 5.0 μl, carry out the reverse transcription reaction on the PCR instrument according to the following conditions: 42°C for 15-30 minutes, 95°C for 5 minutes, after the reaction is completed, ...
Embodiment 3
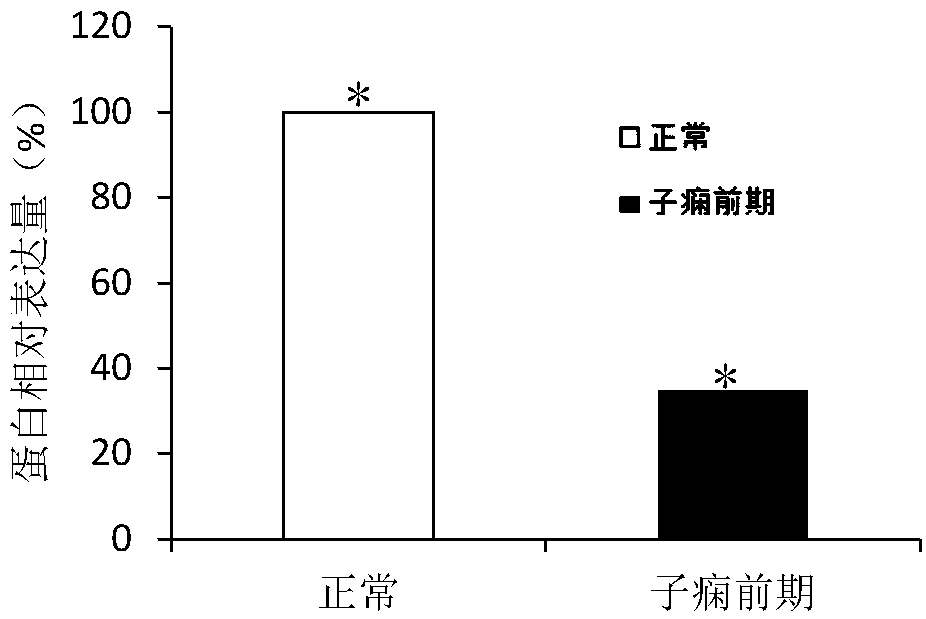
[0095] Example 3 Western blot detection of the expression level of MBOAT1 protein
[0096] 1. Protein sample preparation
[0097] Follow the instructions of the EpiQuik Tissue / Cell Total Protein Extraction Kit for protein extraction.
[0098] 2. Electrophoresis
[0099] β-actin was used as internal reference. 50 μg of total protein was separated by SDS-PAGE, electrotransferred to PVDF membrane, and blocked with 1× TBST containing 5% skimmed milk powder at room temperature for 1 hour; added MBOAT1 monoclonal antibody and β-actin monoclonal antibody respectively, and overnight at 4°C; 1× Wash the membrane 4 times with TBST, add secondary antibody, and incubate at room temperature for 1 h; wash the membrane 4 times with 1×TBST, react in Super Signal chemiluminescent reagent for 2 min, expose the X-ray film in a dark room, and develop and fix it by conventional methods.
[0100] 3. Statistical processing
[0101] The gray value of the protein band was analyzed using Image J so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com