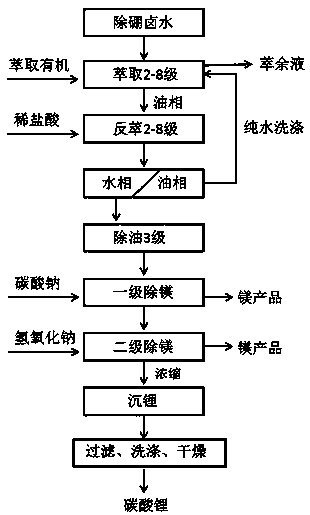
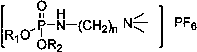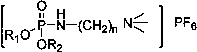Process for extracting lithium in salt lake brine through phosphate ion liquid to produce lithium carbonate
A technology of ionic liquids and phosphate esters, applied in the direction of lithium carbonate;/acid carbonate, process efficiency improvement, etc., can solve problems such as the difficulty of separating and extracting lithium, and achieve easy industrial production and shorten the stratification time , Improve the extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The ionic liquid is dibutyl(2-(trimethyl-amino)ethyl)phosphonic acid hexafluorophosphate, and its structural formula is:
[0029]
[0030] The co-extraction agent is TBP, and the ratio of the two is 1:9; then it is mixed with the diluent hydrogenated kerosene. Add 1 volume of salt lake brine shown in Table 1 into a separatory funnel, add 1 volume of organic phase (compared to O / A=1), wherein the volume ratio of ionic liquid, co-extractant and diluent is 1 : 9: 2, after shaking for 5 minutes, rest and stratify. Determination of Li in the equilibrium aqueous phase + content, the single extraction rate of lithium is calculated to be 75.15%. Add 1 volume of brine to the organic phase, shake for 5 minutes and then separate into layers, and measure the Li in the equilibrium aqueous phase. + content, the calculated extraction rate is 43.99%, continue to repeat, the third yield is 24.67%, the fourth yield is 16.65%, and the fifth yield is 10.08%. After five extractions, ...
Embodiment 2
[0034] The ionic liquid is diethyl(2-(trimethyl-amino)butyl)phosphonic acid hexafluorophosphate, and its structural formula is:
[0035]
[0036] The co-extractant is a mixture of TBP and N503 (N, N'-dimethylheptylacetamide), the volume ratio of which is 1: 10; it is then mixed with diluent hydrogenated kerosene. Add 1 volume of salt lake brine shown in Table 1 into a separatory funnel, add 1 volume of organic phase (compared to O / A=1), wherein the volume ratio of ionic liquid, co-extractant and diluent is 1 : 10: 3, after shaking for 5 minutes, rest and stratify. Determination of Li in the equilibrium aqueous phase + content, the single extraction rate of lithium is calculated to be 73.89%. Add 1 volume of brine to the organic phase, shake for 5 minutes and then separate into layers, and measure the Li in the equilibrium aqueous phase. +content, the calculated extraction rate was 42.80%, and continued to repeat, the third yield was 26.53%, the fourth yield was 17.32%, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com