Osmotic pump controlled release tablet with milnacipran selected-time delayed controlled release function and method for preparing osmotic pump controlled release tablet
A technology of osmotic pump controlled release and milnacipran, applied in the field of milnacipran time-delayed controlled release osmotic pump controlled release tablets and its preparation, can solve the blank period of drug onset, difficult to achieve effective drug controlled release, Low bioavailability and other problems, to reduce the use and environmental pollution and harm to the operator's body, improve bioavailability, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
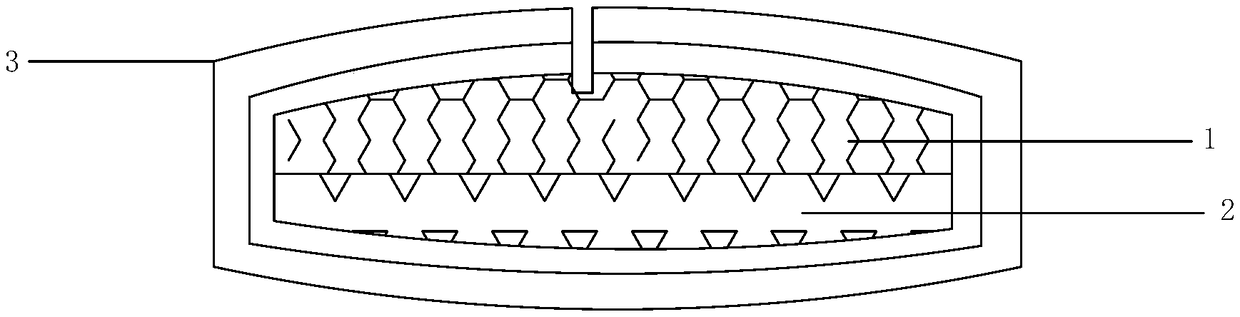
[0028] refer to figure 1 Shown:
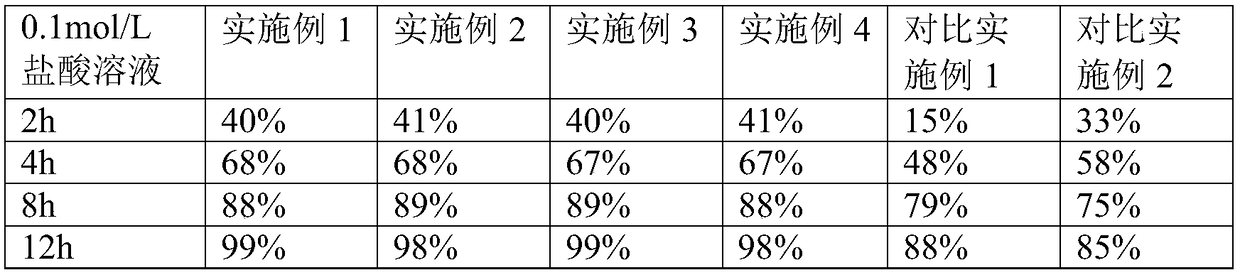
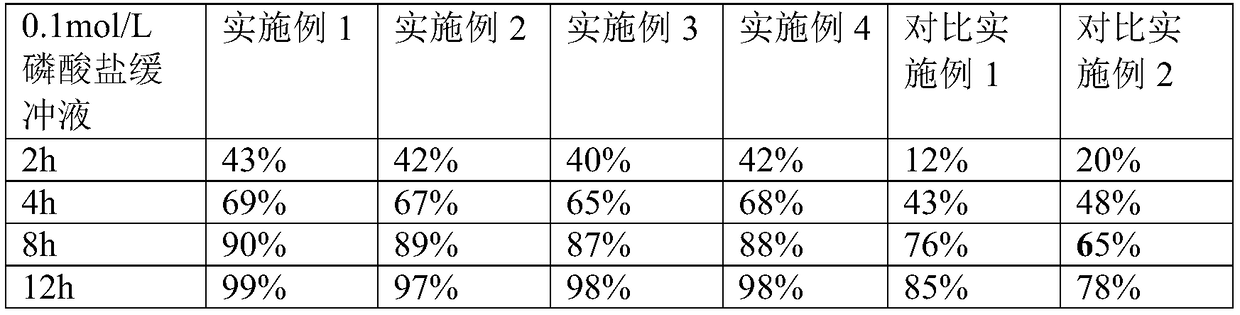
[0029] Preparation of drug-containing layer 1: pass milnacipran, PVPS630, and PEG2000 through a 60-mesh sieve respectively, mix them according to the mass ratio = 8:1:1, and place them in the hopper of the extruder at a speed of 20r / min. The temperature is 120°C, the material is extruded in the shape of strips through the die hole of the machine head, cooled at room temperature, passed through a 40-mesh sieve with a granulator, taken out, and set aside.
[0030] Preparation of push layer 2: Take 75g of polyoxyethylene (PEO, molecular weight: 6 million), 300.5g of povidone k, 8g of sodium chloride, and 2g of iron oxide red, mix well, add ethanol to stir and granulate, and dry at 45°C Dry for 2 hours, let cool to room temperature, take out, sieve with 24 mesh, add 0.42g of magnesium stearate, and mix well.
[0031] Tablet compression: 100 double-layer tablets are compressed by double-layer tablet technology, and the weight of the drug-containi...
Embodiment 2
[0035] The preparation process of this example continues the preparation process of Example 1, the difference is that milnacipran, PVPS630, and PEG2000 are respectively passed through a 60-mesh sieve, and mixed according to the mass ratio = 8:2:1, and the others are the same as in Example 1. same.
Embodiment 3
[0037] The preparation process of this example continues the preparation process of Example 1, the difference is that milnacipran, PVPS630, and PEG6000 are respectively passed through a 60-mesh sieve, and mixed according to the mass ratio = 8:1:1, and the others are the same as in Example 1. same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com