Tripterine-dendritic macromolecular conjugate, preparation method thereof and application
A technology for triptolide and dendrimer, which is applied in the field of triptolide-dendrimer conjugate and its preparation, and can solve the problems of prolonging the systemic circulation time and not having specific targeting to tumor cells, etc. , to reduce toxic side effects, solve poor water solubility, and prolong circulation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
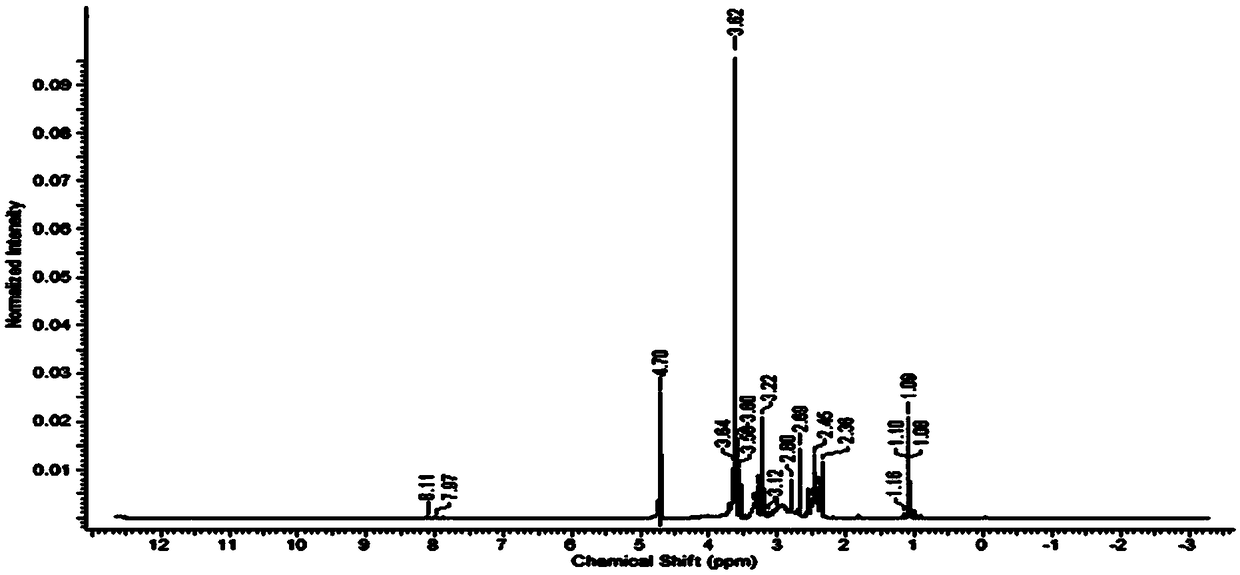
[0055] Example 1: Preparation of tripterine-fifth generation dendrimer conjugate modified by targeting EpCAM aptamer
[0056] (1) Take 20ml of the fifth generation PAMAM (G5-NH 2 ) (5mg / ml) methanol solution, rotate to remove methanol, completely dissolve in N,N-dimethylformamide, add 44.4mg of succinic anhydride in the dark, and stir at room temperature overnight for 24 hours, after the reaction, use a dialyzer with a molecular weight of 3500 ddH 2 Dialyzed against O for two days and then freeze-dried to obtain the G5-COOH polymer.
[0057] (2) Weigh 4.5 mg of G5-COOH polymer and dissolve it in phosphate buffered saline (PBS, pH6.8), add 4.8 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC ), after stirring at room temperature for 15 minutes in the dark, then add 1.4 mg of N-hydroxysuccinimide (NHS), continue stirring at room temperature for 45 minutes, and then add 40.5 mg of NHS 2 -PEG-COOH, stirred at room temperature overnight in the dark, after the reaction, p...
Embodiment 2
[0064] Example 2: Preparation of tripterine-fifth generation dendrimer conjugate modified by targeting EpCAM antibody
[0065] (1) Take 60ml of the fifth generation PAMAM (G5-NH 2 ) (5mg / ml) methanol solution on a rotary evaporator to remove methanol, then completely dissolved in N,N-dimethylformamide, added 133.2mg succinic anhydride in the dark and stirred at room temperature overnight, after the reaction was completed, use Dialysis bag placed in ddH 2 Dialyzed against O for two days and then freeze-dried to obtain the G5-COOH polymer.
[0066] (2) Weigh 3 mg of G5-COOH polymer and dissolve it in phosphate buffered saline (PBS) at pH 6.8, add 14.4 mg of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide ( EDC), after stirring at room temperature for 15min in the dark, then add 4.2mg N-hydroxysuccinimide (NHS), continue stirring at room temperature for 45min, then add 121.5mg NH 2 -PEG-COOH, stirred at room temperature overnight in the dark, after the reaction, put a dialysis b...
Embodiment 3
[0069] Example 3: Preparation of tripterine-sixth generation dendrimer conjugate targeting EpCAM aptamer / antibody modification (1) Take 20ml of the sixth generation PAMAM (G6-NH 2 ) (5mg / ml) methanol solution on a rotary evaporator to remove methanol, then completely dissolved in N,N-dimethylformamide, added 44mg succinic anhydride in the dark and stirred at room temperature overnight, after the reaction was completed, dialyzed with a molecular weight of 3500 ddH 2 Dialyzed against O for two days and then freeze-dried to obtain the G6-COOH polymer. Other steps are the same as above (2), (3), (4).
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com