Method for encapsulating slightly soluble drugs by ZnO-containing hydrogel beads
A technology of insoluble drugs and hydrogel beads, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, capsule delivery, etc., can solve the problems of poor drug stability and low loading rate of insoluble drugs, and achieves simple, The effect of good pH sensitivity and good sustained release performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1 (SA / N-CMCS-ZnO-3)
[0035] This embodiment provides a method for preparing ZnO-containing hydrogel beads, which includes the following steps: first prepare carboxymethyl chitosan-ZnO nanoparticles in situ, and then uniformly add them to an aqueous solution of sodium alginate to chlorinate Calcium acts as a cross-linking agent to form spherical particles. Specific steps are as follows:
[0036] 1g carboxymethyl chitosan was dissolved in 100ml deionized water, heated to 50°C in a magnetic stirrer and stirred for 30min until completely dissolved; 0.595g Zn(NO 3 ) 2 ·6H 2 O was added to the above solution and stirred for 30 minutes; then 50ml of 0.2M NaOH solution was added dropwise, stirred for 2 hours, centrifuged, washed, and dried in an oven at 50°C for 24 hours to obtain ZnO nanoparticles;
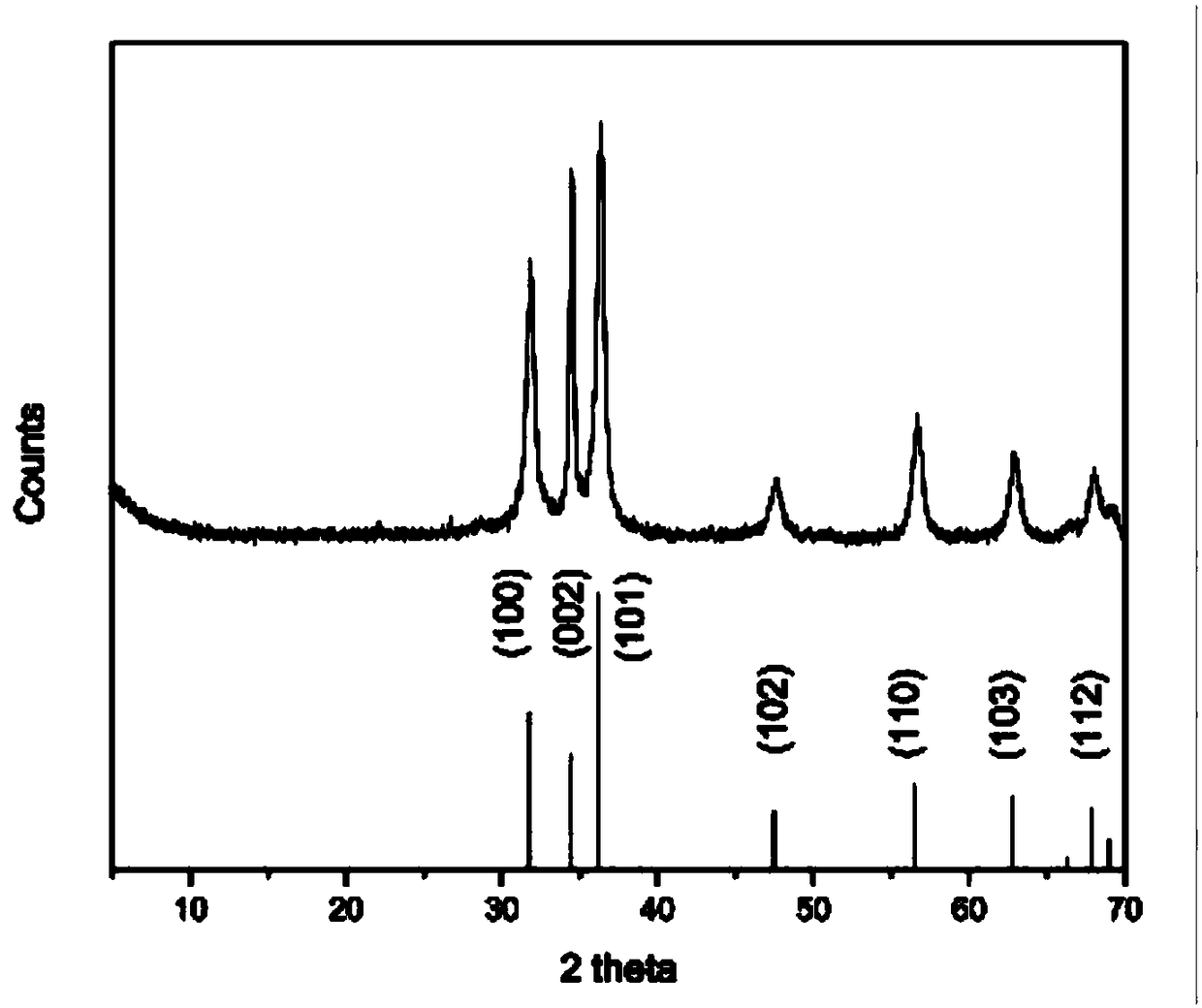
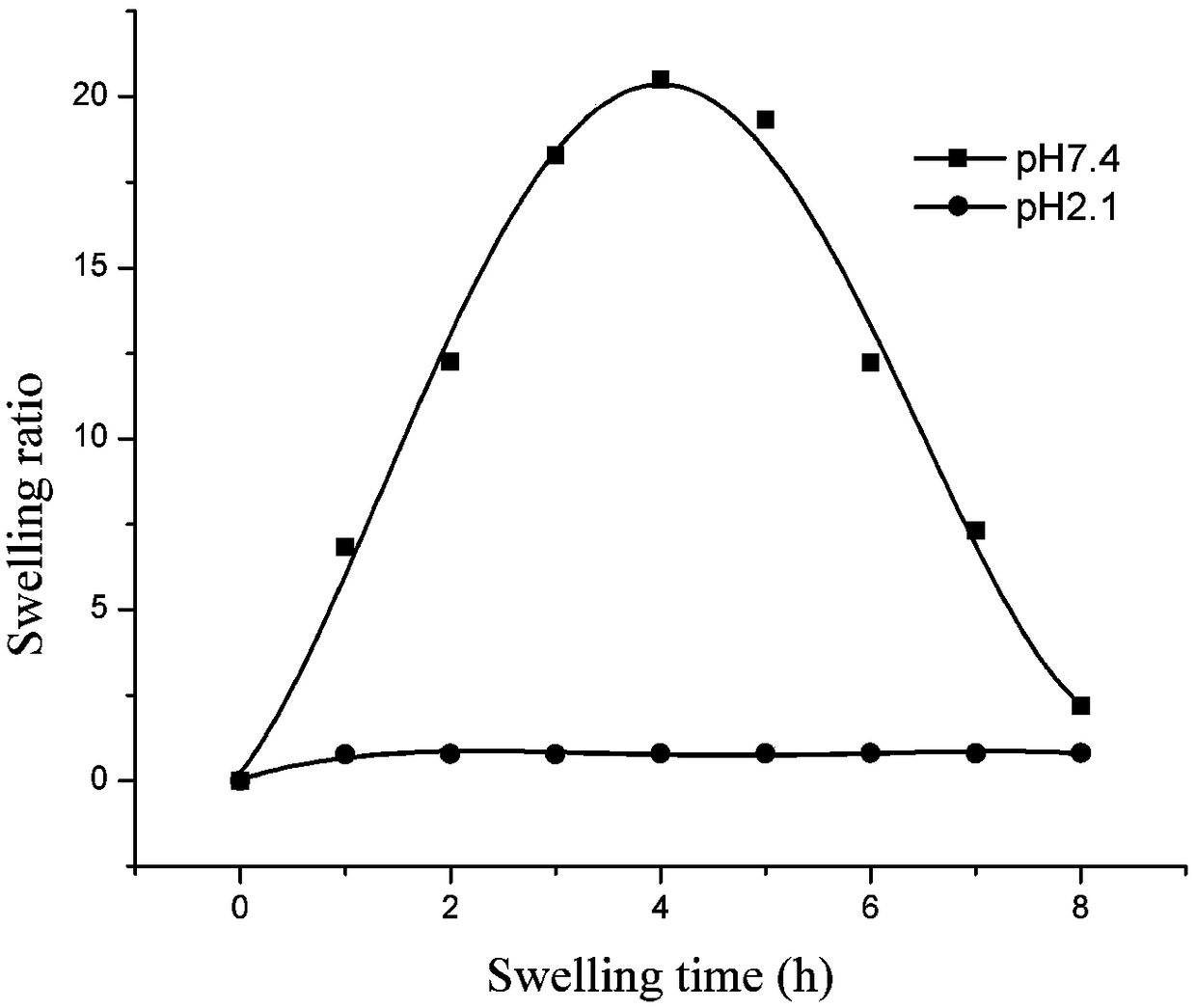
[0037] The prepared ZnO nanoparticles were analyzed by X-ray diffraction and scanning electron microscopy, and the specific results were as follows: figure 1 and f...
Embodiment 2
[0040] Example 2 (SA / N-CMCS-ZnO-1)
[0041] This embodiment provides a preparation method of ZnO-containing hydrogel beads, the specific steps are as follows:
[0042] Dissolve 0.5g carboxymethyl chitosan in 100ml deionized water, heat up to 70°C in a magnetic stirrer and stir for 30min until completely dissolved; 0.595g Zn(NO 3 ) 2 ·6H 2 O was added to the above solution and stirred for 30 minutes; then 50ml of 0.3M NaOH solution was added dropwise, stirred for 2 hours, centrifuged, washed, and dried in an oven at 70°C for 24 hours;
[0043] Add 0.01g of the zinc oxide nanoparticles prepared above evenly into 100ml of deionized water, ultrasonic the probe, the ultrasonic power of the probe is 500W, ultrasonic 3s, intermittent 2s, stir well; then add 2g sodium alginate and stir for 1h until uniform; Add it dropwise into 2% calcium chloride solution, let it stand for 12 hours, rinse the surface of the gel with deionized water, and put the gel in an oven at 37°C to dry for 12 h...
Embodiment 3
[0044] Embodiment 3 (SA / N-CMCS-ZnO-2)
[0045] This embodiment provides a preparation method of ZnO-containing hydrogel beads, the specific steps are as follows:
[0046] Dissolve 2g carboxymethyl chitosan in 100ml deionized water, heat up to 80°C in a magnetic stirrer and stir for 30min until completely dissolved; 0.595g Zn(NO 3 ) 2 ·6H 2 O was added to the above solution and stirred for 30 minutes; then 50ml of 0.1M NaOH solution was added dropwise, stirred for 2 hours, centrifuged, washed, and dried in an oven at 80°C for 24 hours;
[0047] Add 0.03g of the zinc oxide nanoparticles prepared above into 100ml of deionized water, ultrasonic the probe, the ultrasonic power of the probe is 400W, ultrasonic 4s, intermittent 1s, stir evenly; then add 3g sodium alginate and stir for 1h until uniform; the above solution is added drop by drop Add 3% calcium chloride solution, let it stand for 12 hours, rinse the surface of the gel with deionized water, and put the gel in an oven a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com