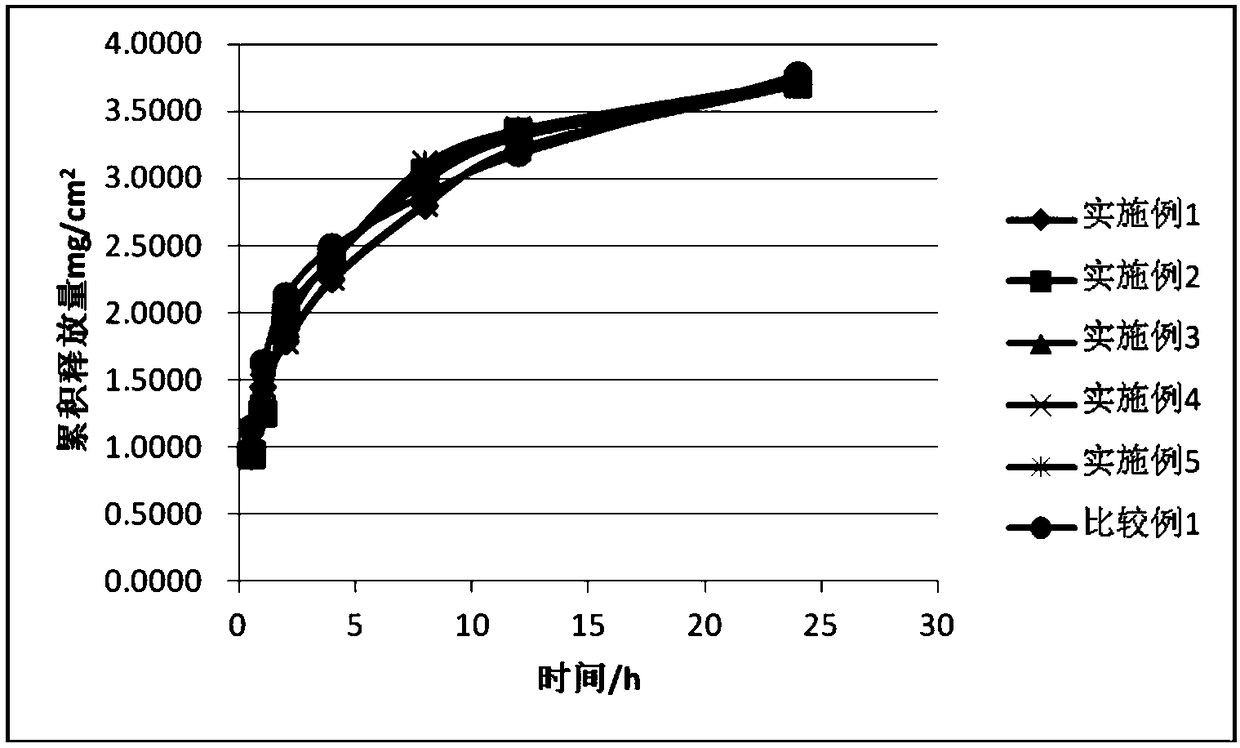
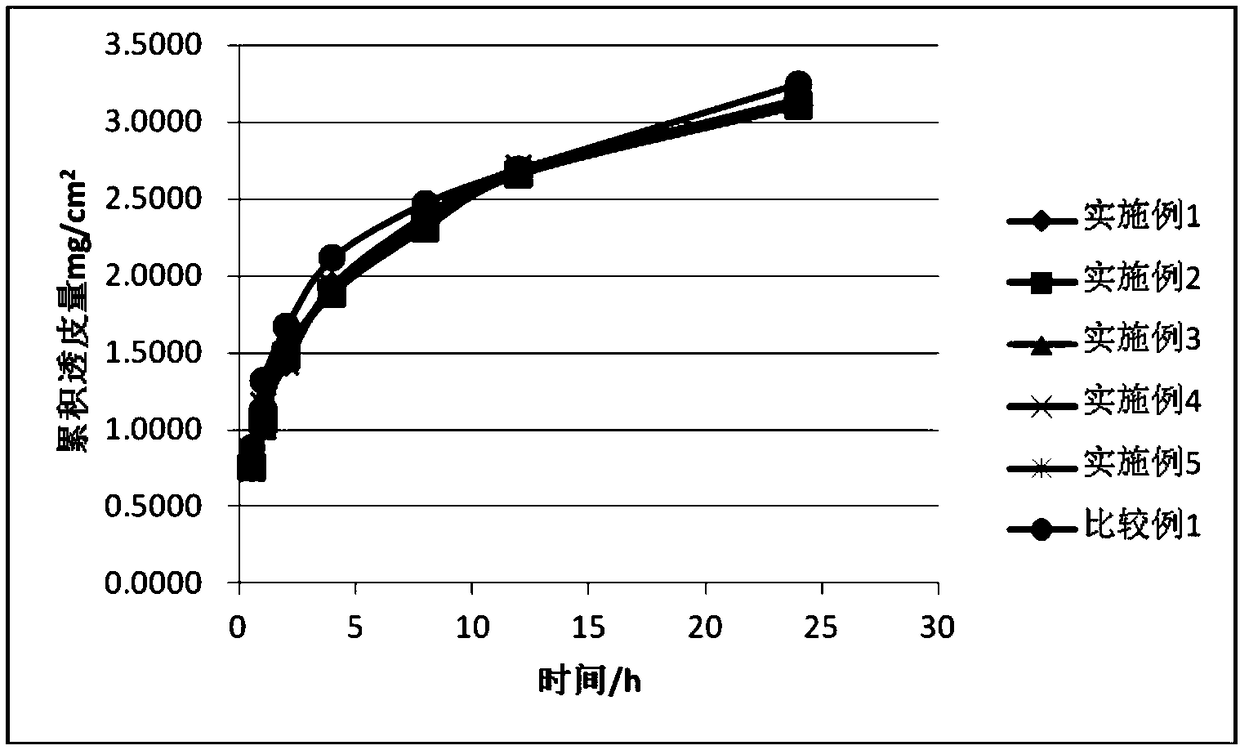
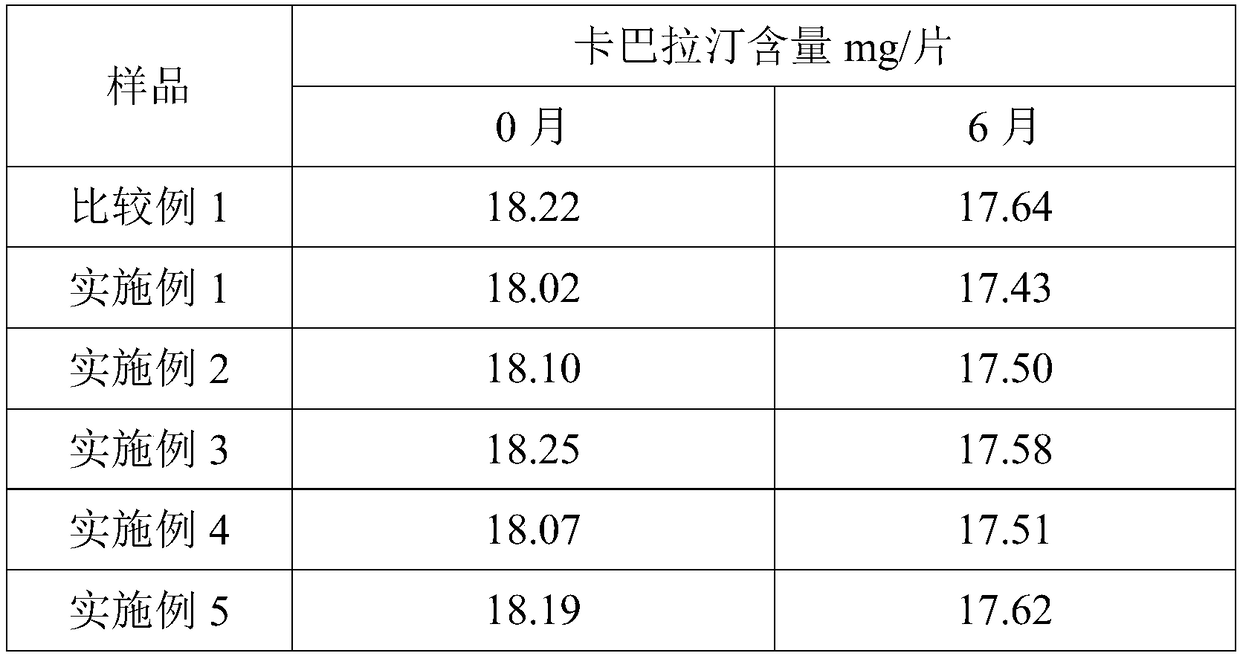
Rivastigmine gel paste and preparation method thereof
A technology of rivastigmine gel plaster and plaster applied in the field of pharmacy, which can solve the problems of poor patient compliance and large skin irritation, and achieve the effects of comfortable application, improved gloss, and increased stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of rivastigmine gel patch
[0029] (1) Weigh 3 parts of carbomer, 0.5 parts of sodium carboxymethylcellulose and 5 parts of povidone K30 respectively, add an appropriate amount of water to fully swell, stir evenly, and then mix evenly to obtain component I;
[0030] (2) Weigh 10 parts of sodium polyacrylate, add glycerin and stir until uniform, add 1 part of vitamin E and stir evenly, add 1 part of rivastigmine, stir evenly, and obtain component II;
[0031] (3) Weigh 0.1 part of disodium edetate and 0.1 part of aluminum glycolate, add water and mix evenly to obtain component III;
[0032] (4) Take an appropriate amount of water, add 0.05 parts of tartaric acid, stir and dissolve to obtain component IV;
[0033] (5) Weigh 0.1 part of ethyl hydroxybenzoate, dissolve it with a small amount of ethanol, add it to component II, then add components I, III, and IV in sequence, and stir for 10 minutes in an airtight, coating, compounding, and cutt...
Embodiment 2
[0034] Embodiment 2: the preparation of rivastigmine gel patch
[0035] (1) Weigh 10 parts of carbomer, 1 part of sodium carboxymethylcellulose and 15 parts of povidone K30 respectively, add an appropriate amount of water to fully swell, stir evenly, and then mix evenly to obtain component I;
[0036] (2) Weigh 30 parts of sodium polyacrylate, add glycerin and stir until uniform, add 3 parts of vitamin E and stir evenly, add 3 parts of rivastigmine, stir evenly, and obtain component II;
[0037] (3) Weigh 0.5 part of disodium edetate and 0.3 part of aluminum glycolate, add water and mix well to obtain component III;
[0038] (4) Take an appropriate amount of water, add 0.2 parts of tartaric acid, stir and dissolve to obtain component IV;
[0039] (5) Weigh 0.3 parts of ethyl hydroxybenzoate, dissolve it with a small amount of ethanol, add it to component II, then add components I, III, and IV in sequence, and stir for 20 minutes in an airtight, coating, compounding, and cutti...
Embodiment 3
[0040] Embodiment 3: the preparation of rivastigmine gel patch
[0041] (1) Weigh 5 parts of carbomer, 0.5 parts of carboxymethylcellulose sodium and 10 parts of povidone K30 respectively, add an appropriate amount of water to fully swell, stir evenly, and then mix uniformly to obtain component I;
[0042] (2) Weigh 20 parts of sodium polyacrylate, add glycerin and stir until uniform, add 2 parts of vitamin E and stir evenly, add 2 parts of rivastigmine, stir evenly, and obtain component II;
[0043] (3) Weigh 0.2 parts of disodium edetate and 0.2 parts of aluminum glycylate in an appropriate amount, add water and mix evenly to obtain component III;
[0044] (4) Take an appropriate amount of water, add 0.1 part of tartaric acid, stir and dissolve to obtain component IV;
[0045] (5) Weigh 0.2 parts of ethylparaben, dissolve it with a small amount of ethanol, add it to component II, then add components I, III, and IV in sequence, and stir for 15 minutes in an airtight, coating, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com