Preparation method of lipid wrapped solid drug nano particle
A nanoparticle and drug technology, applied in the field of biomedicine, can solve the problem of low drug loading of nano drugs, achieve the effect of small batch-to-batch differences and great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The preparation of embodiment 1 methotrexate nanoparticle solution
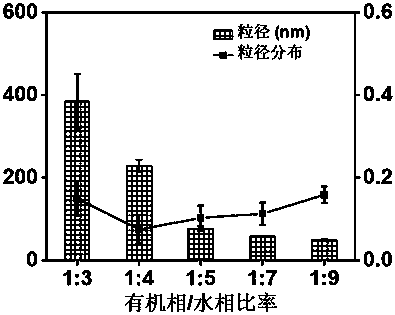
[0033] Weigh methotrexate, dissolve in a mixed solvent of N,N-dimethylformamide and N-methylpyrrolidone (volume ratio 7:3), and prepare a 4 mg / mL organic solution of methotrexate. It is introduced into the first channel of the four-channel vortex mixer, and the other three channels are introduced with ultrapure water to achieve rapid turbulent mixing to prepare the methotrexate nanoparticle solution. The volume ratio of the methotrexate organic solution to the aqueous phase is 1:3-9, respectively, and the flow rate of the organic phase is 6 mL / min.
[0034] When changing the volume ratio of the methotrexate organic solution (organic phase) and the aqueous phase, methotrexate nanoparticles with different particle sizes can be prepared. The specific ratio and particle size relationship is as follows: figure 1 As shown, it can be seen from the figure that when the volume ratio of the organic phase to the...
Embodiment 2
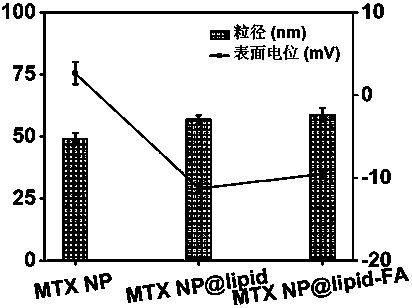
[0037] Example 2 Preparation of nanoparticles of lipid-encapsulated methotrexate
[0038]Weigh 1,2-dioleoyl-SN-glycero-3-phosphoethanolamine (DOPE), cholesterol succinate monoester (CHEMS), 1,2-distearoyl-SN-glycero-3-phosphoethanolamine - Polyethylene glycol (2000) (DSPE-PEG), prepared as a mixture at a molar ratio of 10:9:1, dissolved in dichloromethane to prepare a 10 mg / mL mixed lipid solution for later use. Pipette gun to quantitatively pipette 0.1 mL of mixed lipid dichloromethane solution and add it to 10 mL of 4% ethanol solution, and the solution is sonicated with a probe ultrasonic instrument until it is clear and ready for use. After mixing the lipid ethanol solution of equal volume and the methotrexate nanoparticle solution with a particle diameter of 49.1nm prepared in Example 1, the mass ratio of methotrexate and mixed lipids in the mixed solution was 4:1, and mixed The solution was repeatedly extruded 7 times through a 100-nanometer polyvinylidene fluoride memb...
Embodiment 3
[0045] Example 3 Preparation of lipid-coated chlorambucil or doxorubicin nanoparticles
[0046] Weigh chlorambucil or doxorubicin and dissolve them in dimethyl sulfoxide or N,N-dimethylformamide respectively to make 1 mg / mL or 0.5 mg / mL organic solutions, and introduce them into The first channel of the four-channel vortex mixer, and the other three channels are introduced with ultrapure water to achieve rapid turbulent mixing to prepare the chlorambucil nanoparticle solution and the doxorubicin nanoparticle solution. Among them, the volume ratio of chlorambucil nanoparticle solution (organic phase) or doxorubicin nanoparticle solution (organic phase) to the aqueous phase was set to 1:3-11 respectively, and the flow rate of the organic phase was 10 mL / min. A chlorambucil nanoparticle solution and a doxorubicin nanoparticle solution were obtained.
[0047] Referring to the steps in Example 2, lipid-coated chlorambucil nanoparticles and lipid-coated doxorubicin nanoparticles we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com