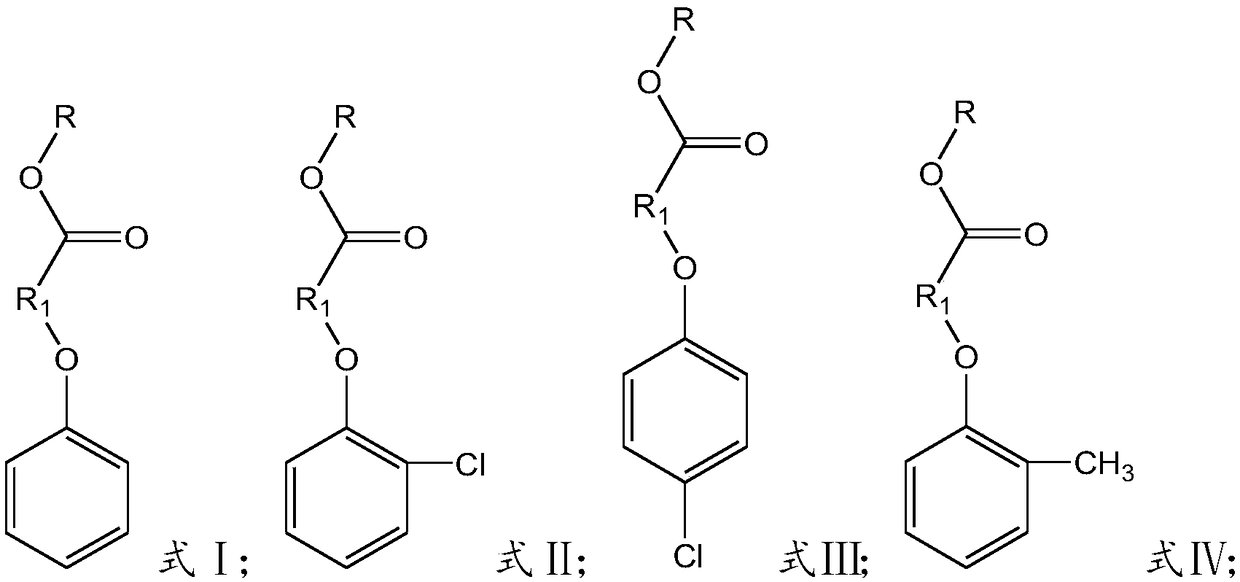
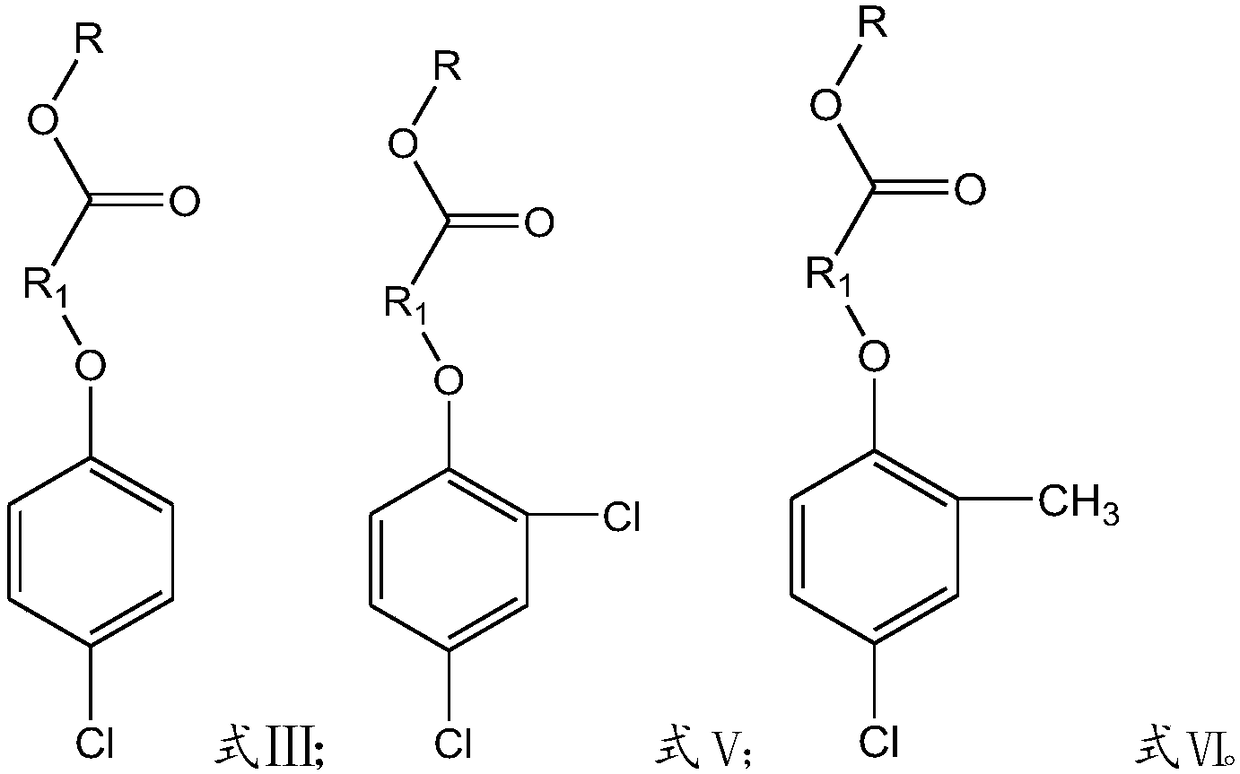
Preparation method of chlorophenoxycarboxamide salt
A technology of chlorinated phenoxycarboxylic acid amine salt and chlorophenoxycarboxylic acid ester, which is applied in the field of herbicide preparation, can solve problems such as environmental hazards, loss of active ingredients, and high pressure of three wastes treatment, so as to improve production capacity and increase Utilization efficiency and energy saving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Add 167.87g 99% methyl phenoxyacetate (1mol), 1.26g 99% zinc chloride and 1.68g 99% 2,4,6-trimethyldiphenylsulfide successively in a 500mL four-necked bottle, at - 154.69g of 99% chlorine gas (2.16mol) was introduced at 20°C, and the heat preservation reaction was completed for 30 minutes, and the distilled under 1kpa pressure and collected fractions at 140-150°C to obtain 236.21g of methyl 2,4-dichlorophenoxyacetate , the content is 98.8%, and the yield is 99.28% based on methyl phenoxyacetate.
[0065] After testing, the impurity content is as follows: 0.03% of methyl 4-chlorophenoxyacetate, 0.09% of methyl 2,6-dichlorophenoxyacetate, 0.16% of methyl 2,4,6-trichlorophenoxyacetate, The content of 2,4,6-trimethyldiphenylsulfide is 0.13%.
[0066] Add 118.63g of 40% dimethylamine to the distilled 2,4-dichlorophenoxyacetic acid methyl ester, react at 130°C and distill off the generated alcohol to obtain 2,4-dichlorophenoxyacetic acid dimethylamine Salt 319.14g, content ...
Embodiment 2
[0074] Add 196.21g 99% propyl phenoxyacetate (1mol), 0.29g 99% ferric chloride and 0.69g 99% 2-methyldiphenylsulfide successively in a 500mL four-necked flask, add 137.70 g 99% sulfuryl chloride (1.01mol), after the dropwise addition, keep the temperature for 30 minutes, distill and collect the fraction at 145-155°C under the pressure of 1kpa to obtain 229.01g of propyl 4-chlorophenoxyacetate, the content is 98.9%, the yield 99.04% based on propyl phenoxyacetate.
[0075] Add 129.51g of 40% dimethylamine to the distilled propyl 4-chlorophenoxyacetate, react at 120°C and distill off the resulting alcohol to obtain 291.58g of dimethylamine 4-chlorophenoxyacetate with a content of 78.4 %, the yield is 98.64% based on propyl phenoxyacetate.
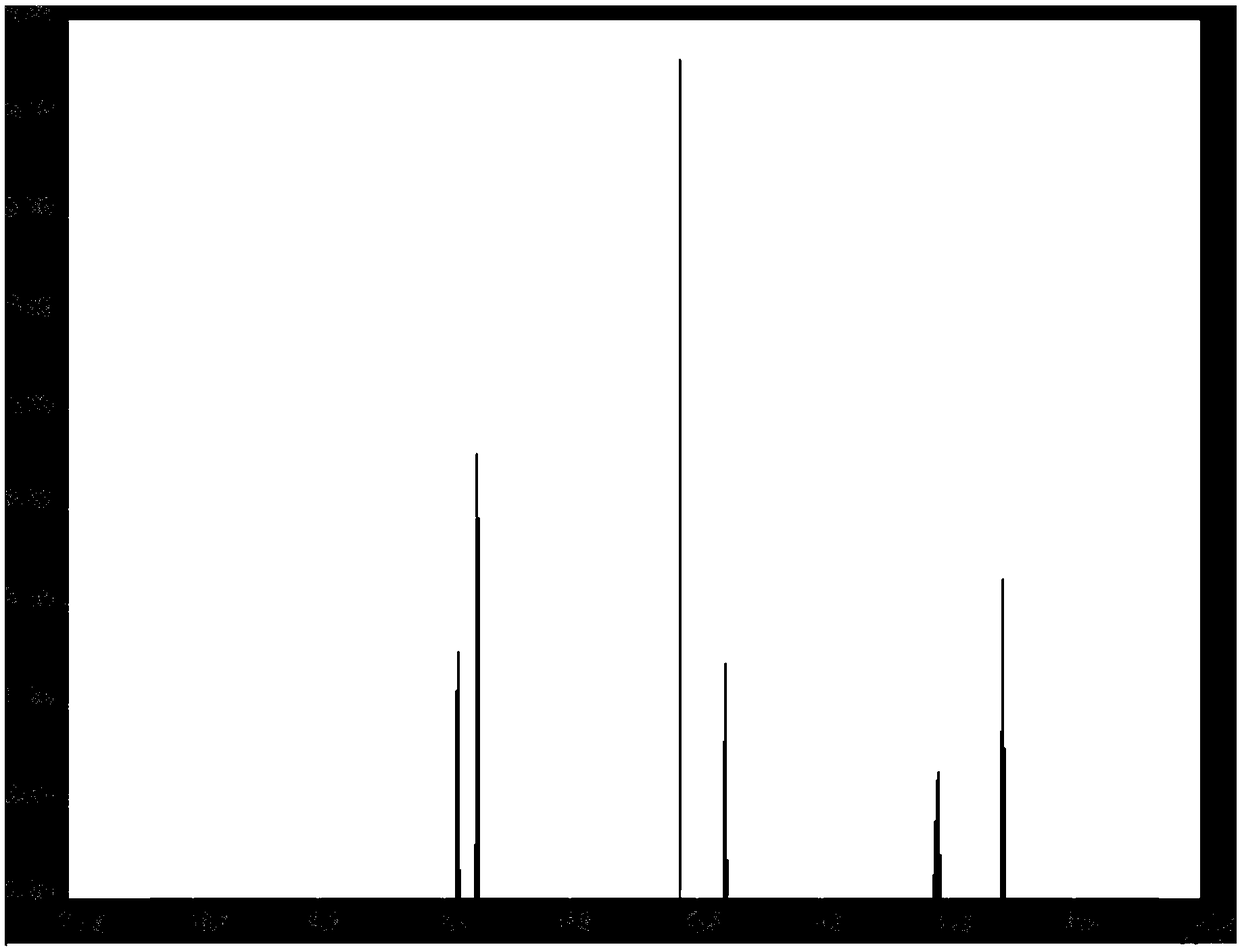
[0076] Adopt nuclear magnetic resonance to detect the propyl 4-chlorophenoxyacetate of preparation, the results are shown in figure 1 , figure 1 Propyl NMR spectrum of 4-chlorophenoxyacetate.
Embodiment 3
[0078] Add 196.21g 99% methyl phenoxybutyrate (1mol), 1.08g99% titanium tetrachloride and 0.88g 99% 2,4,6-trimethyldiphenylsulfide successively in a 500mL four-necked flask, 157.56g of 99% chlorine gas (2.2mol) was introduced at 0°C, and the heat preservation reaction was completed for 30 minutes. Distilled at a pressure of 1kpa and collected at 150-160°C to obtain methyl 2,4-dichlorophenoxybutyrate 264.04g, content 98.8%, yield 99.14% based on methyl phenoxybutyrate.
[0079] Add 72.94g of 25% ammonia water to the distilled methyl 2,4-dichlorophenoxybutyrate, react at 100°C and distill off the resulting alcohol to obtain 2,4-dichlorophenoxybutyric acid ammonium salt 303.00 g, the content is 86.7%, and the yield is 98.74% based on methyl phenoxybutyrate.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com