Keratinase mutant subjected to thermal stability modification
A kind of keratinase mutation and keratinase technology, which is applied in the field of keratinase mutants, and can solve the problem that the thermal stability of strains is not suitable for industrial applications and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: site-directed mutagenesis and construction of recombinant bacteria
[0031] The highly flexible loop region is often the part of the enzyme molecular structure that is unstable to heat and is prone to unfolding first. In this study, the amino acid sequence was selected as SEQ ID NO: 2. Eight key amino acid sites on the highly flexible loop region of keratinase (S78L, L96T, N118S, N123Y, S173R, N218S, M222A, S236C) for mutation transformation research.
[0032] Construction of keratinase site-directed transformation mutants by one-step reverse PCR method, figure 1 Shown are the PCR verification results of each mutant colony, and the band at 1800 bp in the figure is consistent with the size of the target fragment amplified with the plasmid universal primers. After further sequencing verification, the correctly constructed plasmid for site-directed mutagenesis was transformed into host cells.
Embodiment 2
[0033] Embodiment 2: thermal stability of mutant recombinant bacteria
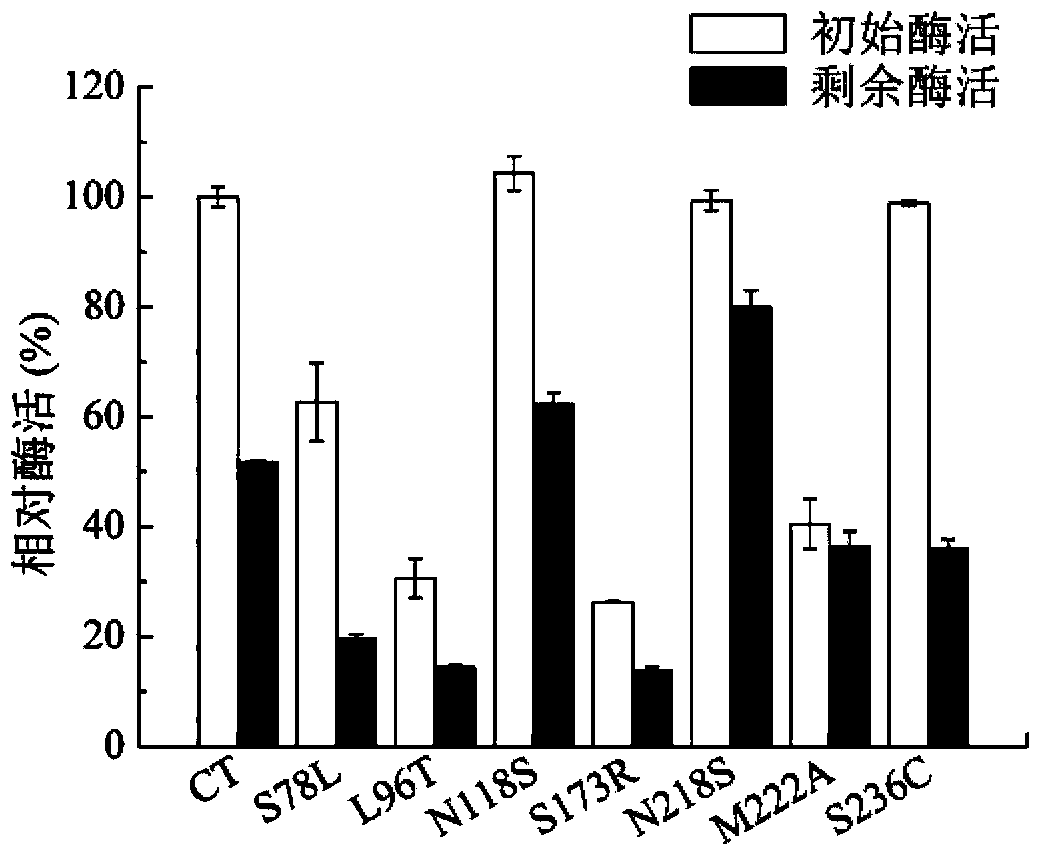
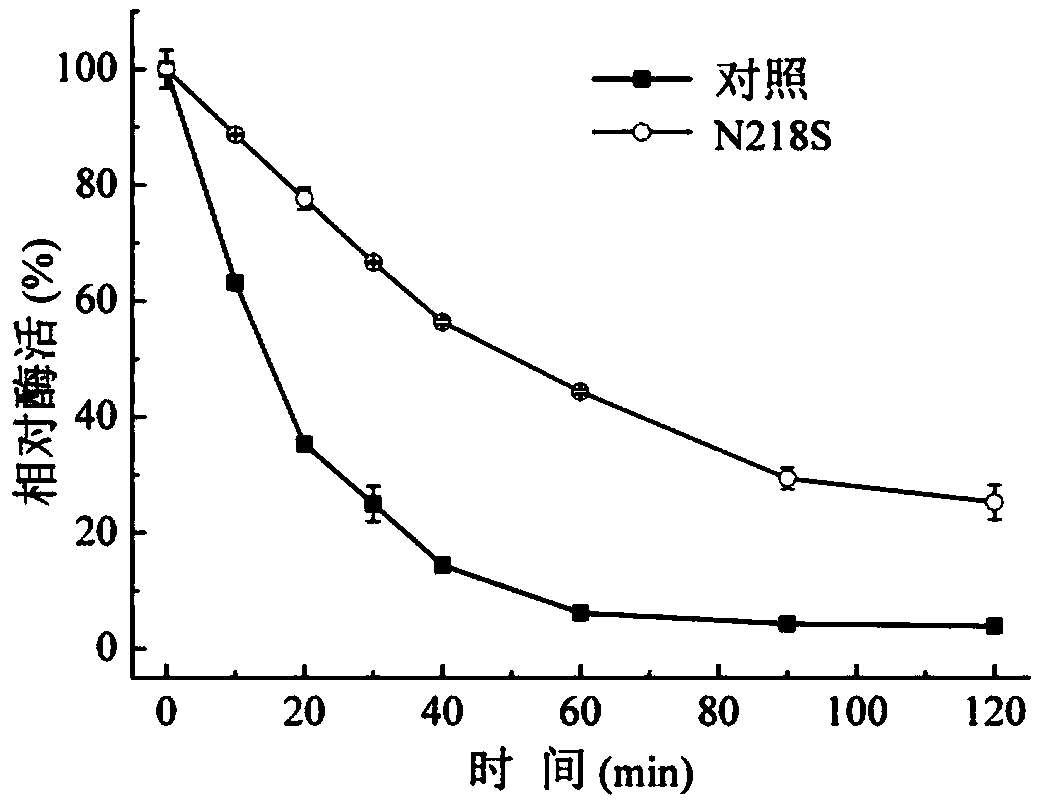
[0034] Stratify the correctly constructed recombinant bacteria at each mutation site to isolate a single colony, pick a single colony and culture it in LB medium (Kan) for 8-12 hours, and inoculate it into TB fermentation medium (Kan) with 2% inoculum After 30 hours of fermentation in medium, the fermentation supernatant, i.e. each mutant recombinant enzyme, was heat-treated at 60° C. for 15 minutes, and the enzyme activities before and after heat treatment were detected respectively. The activity of the original enzyme that was not mutated and not heat-treated at 60°C was taken as 100%. The result is as figure 2 As shown, only the three mutants, N118S, N218S, and S236C, had the same enzyme activity levels as the initial control bacteria, and the rest showed a decrease in enzyme activity. After heat treatment, the remaining enzyme activities of N118S and N218S mutant enzymes were higher than those of th...
Embodiment 3
[0037] Embodiment 3: keratinase degradation feather degradation situation analysis
[0038]Ferment the recombinant bacteria with improved thermostability of the modified keratinase to produce enzyme under the condition of optimized medium, and prepare 1000U / mL feather degrading enzyme solution, then take 60mL feather degrading enzyme solution and add it to 0.6g feather meal In a 250mL Erlenmeyer flask, the reaction system was placed at 40°C and 220rpm for reaction, and the degradation of the feathers was observed at regular intervals, and samples were taken to detect the components of the reaction solution. The results showed that after 48 hours of reaction, keratinase showed a good feather degradation effect, gradually degrading from the initial intact feathers to fine fibers, and the stems of feathers were difficult to degrade, but were obviously thinner than the initial stems. Observed by scanning electron microscope, the situation before and after feather degradation is as...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com