Quantitative detection kit for hepatitis B viral nucleic acid
A hepatitis B virus, detection kit technology, applied in the direction of microbial determination/inspection, microorganisms, biochemical equipment and methods, etc., can solve the problem of long amplification time, achieve high amplification efficiency, easy storage and transportation, The effect of improving convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1 The composition of kit of the present invention
[0026] 1. PCR reaction solution 1 20µl, which contains 4 specific primers (SEQ ID No.1~4) and 2 specific probes (SEQ ID No.5~6), the primers of SEQ ID No.1~4 The concentration is 25 pmol / µl, the concentration of probe SEQ ID No.5~6 is 15 pmol / µl; 20mM dNTP, 10×PCR reaction buffer, 10U of bifunctional enzyme, 0.4U of UNG enzyme, 1.1µl of enzyme protection solution.
[0027] 2. PCR reaction solution 2 10µl: contains 25mM MnCl 2 , 0.09% sodium azide.
[0028] 3. DNA internal control: artificially constructed plasmid, the concentration is 1.0E+05-1.0E+06copies / ml.
[0029] 4. Quantitative reference products A, B, C, and D: all are pseudoviruses containing the target fragment of HBV.
[0030] 5. Negative quality control: HBV negative serum or plasma,
[0031] 6. Strong positive quality control and critical positive quality control: a pseudovirus containing the target fragment of HBV.
Embodiment 2
[0032] The detection of embodiment 2 HBV DNA
[0033] 1. Build a reaction system
[0034] Calculate the number of samples required, and increase the amount of reagents in proportion to the amount in the table below. Only one copy of strong positive quality control, critical positive quality control, and negative quality control is made.
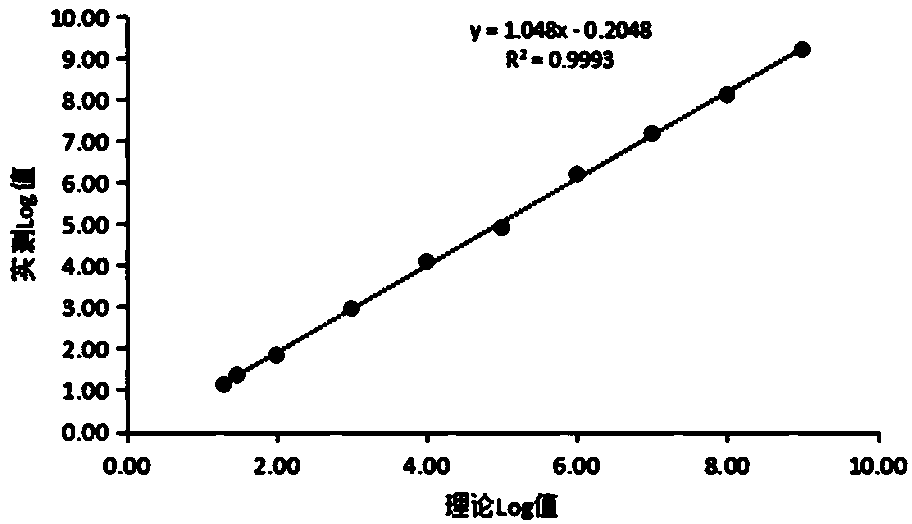
[0035]
[0036] 2. On-board inspection
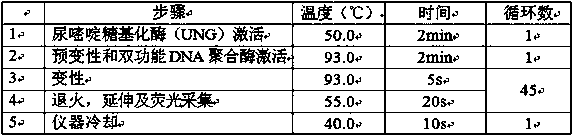
[0037] Directly use the fluorescent quantitative PCR instrument for detection, the amplification program is set to 50°C for 2min, 93°C for 2min, (93°C for 5s, 55°C for 20s) 45Cycle, 40°C for 10s; collect FAM and ROX fluorescence after each cycle.
[0038] 3. Quality control
[0039] Each batch of tests should contain a strong positive control, a borderline positive control, and a negative control, all controls should be unmarked and: Positive controls: 1.0E+05~1.0E+07 IU / mL; critical positive quality control: Ct value should be <45; negative quality control: No Ct in FAM channel, Ct value in RO...
Embodiment 3
[0042] Embodiment 3 The minimum detection limit of the kit of the present invention
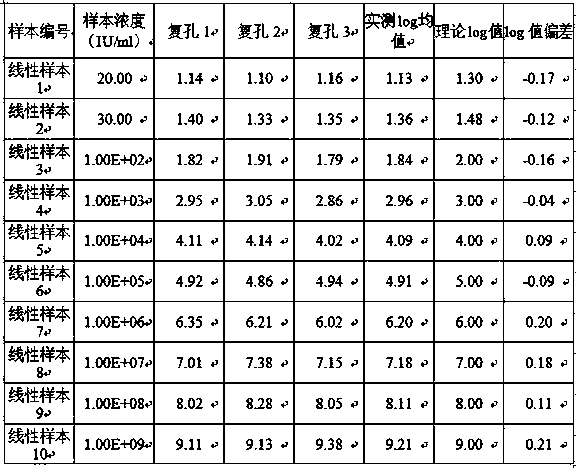
[0043] Use nucleic acid-negative plasma to dilute the reference product with the lowest detection amount in the national reference product (code: 300009-201602) to 5IU / ml, and use the nucleic acid extraction and purification reagent (magnetic bead method) of Zhengzhou Antu Bioengineering Co., Ltd. to perform type B Hepatitis virus DNA was extracted and purified, and tested using this kit, repeated 20 times. The test results are shown in Table 1.
[0044] Table 1
[0045]
[0046] It can be seen from Table 1 that the minimum detection limit of this kit can reach 5 IU / ml when 20 repeated tests are performed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com